Abstract
242160
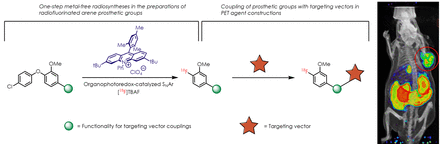
Introduction: 18F-labeled prosthetic groups are important radiopharmaceutical ingredients as they can be conjugated to biologically active ligands in PET agent constructions. Prosthetic groups with [18F]fluoroarene motifs are especially appealing due to the lower tendency of defluorination under physiological environment. However, the conventional arene radiofluorination conditions are usually too harsh for highly reactive conjugation functionalities. The previously established radiosyntheses of [18F]fluoroarene prosthetic groups either employ multi-step radiosyntheses, or use transition-metal reagents. Here we report a one-step metal-free organophotoredox-catalyzed radiofluorination strategy that can synthesize [18F]fluoroarene prosthetic groups with various robust conjugation functionalities. We also report the application examples of these prosthetic groups in PET agent constructions and the imaging studies.
Methods: Organophotoredox-catalyzed nucleophilic aromatic substitution (SNAr) reactions can generate highly reactive radical cation intermediates under mild conditions. The high reactivity of radical cations enabled the efficient radiofluorination process, and the mild reaction conditions offered functional group compatibility with robust conjugation functionalities that wouldn’t survive conventional radiofluorination conditions. We prepared several precursors with unprotected conjugation functionalities, and applied these compounds to our photoredox radiolabeling condition promoted by the mesityl acridinium photocatalyst and 450 nm laser irradiation. Slightly modified radiolabeling conditions generated better results for base-sensitive and volatile radio products. The isolated 18F-labeled prosthetic groups were conjugated to various biologically active ligands, and the afforded PET agents were subjected to small animal imaging studies.
Results: [18F]fluoroarene prosthetic groups with azide, tetrazine, benzyl bromide, chloroacetyl carbamate, and N-succinimidyl ester coupling sites were synthesized with moderate to excellent RCY via our one-step radiosynthesis method. Modifications were carried out to accommodate the sensitive functional groups, achieving higher RCY. We also report the first examples of isocyanate and isocyanide prosthetic groups with [18F]fluoroarene motif via our method. The prosthetic groups with N-succinimidyl ester, benzyl bromide and isocyanate coupling sites were tested in conjugation reactions with various ligands, generating multiple PET agents.
Conclusions: We have developed a one-step metal-free approach in preparing robust prosthetic groups with physiologically stable [18F]fluoroarene motif. Higher step-economy and overall efficiency in radiosyntheses were achieved by applying the mild and highly reactive organophotoredox-catalyzed SNAr reaction. The generated prosthetic groups demonstrated utility in PET agent constructions.