Abstract
2874
Introduction: Chimeric antigen receptor T cells (CAR T) therapy has been playing an important role in the hematological malignancies, but some formidable challenges still exist in the treatment of solid tumors. There are urgent needs for long-term, repeated, and quantitative methods for evaluation of the distribution, activities, metabolism about CAR T treatment in solid tumors. The reporter gene without toxicity and immunogenicity guarantees to accomplish all above goals. Targeting transferrin receptor (TfR) in tumor cells with truncated prostate specific membrane antigen (ΔPSMA) as the reporter gene, we constructed TfR-ΔPSMA-CAR T cells to address the unsatisfied dilemma in this field.
Methods: On the basis of constructing TfR-ΔPSMA-CAR T cells, we launched the preliminary experiments by using the 68Ga -PSMA-617 to visualize and quantify CAR T cells in vitro and in vivo. In the cellular uptake experiment, 1×106 blank T cells or CAR T cells per well were incubated with 68Ga-PSMA-617 for 30 and 60 min to evaluate the binding specificity of the probe to cells (n=6). Different quantity gradients (0, 1×103, 9×103, 9×104 and 9×105) of CAR T cells in 100μL 50% Matrigel were inoculated on both shoulders of Balb/c nude mice. Mice were injected with 5.55 MBq of 68Ga-PSMA-617 via the tail vein and PET/CT scans were conducted at 30 and 60 min after injection.
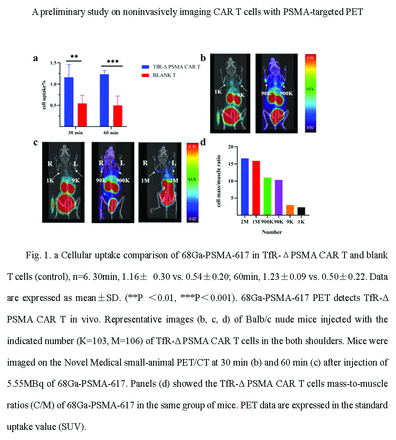
Results: The cellular uptake of 68Ga-PSMA-617 by TfR-ΔPSMA CAR T in vitro was significantly higher than that of control blank T cells at 30 min and 60 min (Fig.1a), indicating that TfR-ΔPSMA CAR T cells were better able to bind to 68Ga-PSMA-617 in vitro. In vivo PET/CT images showed that the PET signal intensity was correlated with the number of inoculated TfR-ΔPSMA CAR T cells (Fig1.b, c, d). Early imaging at 30 min after injection revealed that 9×105 CAR T cells were clearly visible (SUV=0.18), and the minimum number that can be recognized at this time point was 9×103 (Fig.1b). The results of cell mass/muscle uptake ratios were consistent with the PET images, with a ratio of 17 for 200,000 CAR T cells and just 2 for 1,000 CAR T cells (Fig1.c, d).
Conclusions: We successfully constructed TfR-ΔPSMA CAR T cells targeting cellular transferrin receptor (TfR) with truncated prostate specific membrane antigen truncated body (ΔPSMA)as the reporter gene. Our findings preliminarily confirmed that 68Ga-PSMA-617 could sensitively detect TfR-Δ PSMA CAR T cells in vitro and in vivo, providing a new molecular imaging method for future CAR T cell treatment monitoring and patient management.