Abstract
4025
Introduction: PSMA radiotheranostic improves metastatic and relapsing prostate cancer (PC) diagnosis and therapy. The 68Ga/177Lu-PSMA-617 pair offers promising potential but both radioligands could still be improved (e.g. through increased imaging time window or lower kidney uptake). We suggested that 64Cu (β+, β−, Auger electrons; 12.7h half-life) could act as a theranostic radioisotope. We developed the radioligand 64Cu-DOTHA2-PSMA (Fig. 1A) and, based on its stability, hypothesized its efficient use for PC theranostic. Here, we look over the journey of its preclinical study, with the objectives to determine 64Cu-DOTHA2-PSMA’s ability to image and treat PC.
Methods: DOTHA2-PSMA was labelled with 64Cu(OAc)2. Cellular assays included competition binding and cellular kinetics (uptake, internalization, efflux) in LNCaP cells. Balb/c mice were used to assess in vivo stability (n=7, ad 24h post-injection (p.i.)) and biodistribution (n=31, ad 48h p.i.). 1h dynamic PET/CT and static PET/CT (4h and 24h p.i.) were obtained with LNCaP tumor-bearing NRG mice (n=8, including 3 receiving blocking co-injection of natCu-DOTHA2-PSMA). Therapy survival assays were conducted and compared to 177Lu-PSMA-617 and natCu-DOTHA2-PSMA (control) using the same animal model. Toxicity was assessed through weight measurement, 18 days red blood cells follow-up (comparison to normal value obtained with same protocole) and pathological examination of liver, kidneys, salivary gland and tumor (comparison to contorl and non-treated mice). Finally, dosimetry extrapolation to human was obtained based on biodistribution results using OLINDA/EXM 2.1.1.
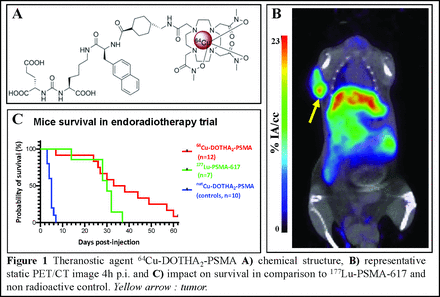
Results: 64Cu-DOTHA2-PSMA was >99% radiolabeled in 5 to 10 min at room temperature with molar activities of 116±30 MBq/nmol. In vitro results showed IC50 = 64±50 nM in competition to PMPA-2 and cellular uptake and internalization respectively up to 34.5±13.6 and 34.1±4.9 %IA/106 cells over 48h p.i. 64Cu-DOTHA2-PSMA was stable in vivo up to 24h. Biodistribution showed low kidney uptake (< 15.8±7.0 %IA/g), but higher liver uptake (maximal at 30.2±4.2 %IA/g at 4h p.i.). PET demonstrated high tumoral uptake up to 24h p.i. (maximal at 23.8±11.5 %IA/cc at 4h p.i.), significantly decreased by blocking injection (p<0.05) (Fig. 1B). In therapy, survival was significantly longer using 64Cu-DOTHA2-PSMA (n=12) than with controls (n=10, p<0.001) (Fig. 1C). It was also longer than with 177Lu-PSMA-617 (n=7), but the difference was not significant (p=0.09). One mouse treated by 177Lu-PSMA-617 reached the maximal allowed weight loss. Red blood cell counts were also significantly lower than normal at days 8 and 12 p.i. with 177Lu-PSMA-617 (p<0.0001), while no significant difference from normal observed with 64Cu-DOTHA2-PSMA. Pathological analysis showed mild, non-pathological fibrosis in kidneys, liver and salivary gland in all survival assays groups, without significant difference between groups. Small, but significant differences were noted between survival assays groups and non-treated controls (p < 0.01) (exceptions: 177Lu-PSMA-617 treated group for kidneys and salivary gland). In tumor, changes such as necrosis, hemorrhage and fibrosis were present, with significantly smaller proportion of alive tumor cells in 64Cu-DOTHA2-PSMA and 177Lu-PSMA-617 treated mice than non-treated controls (p= 0.04 and 0.05, respectively). Finally, human estimated effective dose was 3.1x10-2 mSv/MBq (95% CI: 2.7x10-2– 3.6x10-2).
Conclusions: 64Cu-DOTHA2-PSMA first showed high potential as a PC theranostic agent, thanks to its easy and efficient synthesis and its high in vivo stability. Results then showed that 64Cu-DOTHA2-PSMA offered a long imaging time window and efficiency for radionuclide therapy with acceptable toxicity and dosimetry. In the whole picture, 64Cu-DOTHA2-PSMA is a promising agent for PC theranostic.