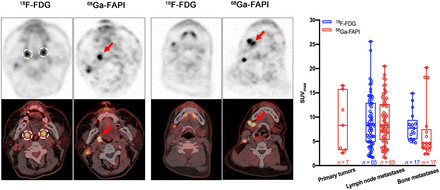
Visual Abstract
Abstract
18F-FDG PET/CT plays an important role in locating the primary tumor for patients with head and neck cancer of unknown primary (HNCUP). Nevertheless, in some cases it can be challenging to locate the primary malignancy on 18F-FDG PET/CT scans. Because 68Ga-radiolabeled fibroblast activation protein inhibitor (FAPI) PET/CT has promising results in detecting different tumor entities, our study aimed to evaluate the performance of 68Ga-FAPI PET/CT for detecting the primary tumor in HNCUP patients with negative 18F-FDG findings. Methods: Eighteen patients (16 men and 2 women; median age, 55 y; age range, 24–72 y) with negative 18F-FDG findings were enrolled in this study. All patients underwent 18F-FDG and 68Ga-FAPI PET/CT within 1 wk. Biopsy and histopathologic examinations were performed in the sites with positive 68Ga-FAPI PET/CT findings. Results: 68Ga-FAPI PET/CT detected the primary tumor in 7 of 18 patients (38.89%). Among these 7 patients, primary tumor sites included the nasopharynx (n = 1), palatine tonsil (n = 2), submandibular gland (n = 2), and hypopharynx (n = 2). The primary tumors showed moderate to intensive uptake of 68Ga-FAPI (mean SUVmax, 8.79; range, 2.60–16.50) and excellent tumor–to–contralateral normal-tissue ratio (mean SUVmax ratio, 4.50; range, 2.17–8.21). In lesion-based analysis, 65 lymph nodes and 17 bone metastatic lesions were identified. The mean SUVmax of lymph node metastases was 9.05 ± 5.29 for 18F-FDG and 9.08 ± 4.69 for 68Ga-FAPI (P = 0.975); the mean SUVmax of bone metastases was 8.11 ± 3.00 for 18F-FDG and 6.96 ± 5.87 for 68Ga-FAPI (P = 0.478). The mean tumor-to-background ratios of lymph node and bone metastases were 10.65 ± 6.59 versus 12.80 ± 8.11 (P = 0.100) and 9.08 ± 3.35 versus 9.14 ± 8.40 (P = 0.976), respectively. Conclusion: We present the first evidence, to our knowledge, of a diagnostic role of 68Ga-FAPI PET/CT in HNCUP. Our study demonstrated that 68Ga-FAPI PET/CT has the potential to improve the detection rate of primary tumor in HNCUP patients with negative 18F-FDG findings. Moreover, 68Ga-FAPI had a performance in assessing metastases similar to that of 18F-FDG.
Head and neck cancer of unknown primary (HNCUP) is defined as a metastatic disease in the cervical lymph nodes with an unidentifiable primary tumor (1), even after a thorough diagnostic workup according to the National Comprehensive Cancer Network (2) and American Society of Clinical Oncology guidelines (3). HNCUP constitutes 1%–5% of all head and neck cancers (4,5). Squamous cell carcinoma (SCC) is the most common pathologic type of HNCUP, and approximately 90% of these cases are associated with human papillomavirus (1). The most frequent primary site of HNCUP is the oropharynx, accounting for 80%–90% (6). However, factors such as small tumor volume, hidden location, slow growth rate, and tumor involution hinder primary site identification (7). The absence of primary tumor identification may result in uncertain treatment decisions and increasing psychologic burden for patients with HNCUP (8).
Medical imaging plays an important role in oncology, particularly in tumor location (9). Conventional imaging modalities, CT and MRI, can provide plentiful anatomic information about primary and metastatic malignancies. However, the detection rates of the primary site for these 2 imaging modalities range from 9% to 23% in HNCUP (10–12). PET/CT, a typical molecular imaging modality, outperforms CT and MRI in identifying the primary tumor, with a detection rate of 25%–69% using 18F-FDG (13–16). Nevertheless, some limitations hamper the application of 18F-FDG PET/CT in primary tumor identification for HNCUP (17,18). First, physiologic 18F-FDG uptake can be seen in any lymphatic structure (especially Waldeyer’s ring), salivary glands, and brown fat. Second, uptake in the symmetric vocal cords and neck muscles is commonly seen if the patient talks or coughs during the uptake period. Third, infection and chronic inflammation (e.g., nasopharyngitis, amygdalitis, and gingivitis) can also result in high 18F-FDG uptake. These limitations may lead to false-positive findings, with a rate of 16%–25% (4,13,16). Last, false-negative 18F-FDG uptake can be seen in small, mucinous, well-differentiated, and necrotic lesions (18). Therefore, novel specific radiopharmaceuticals with low background uptake in the head and neck, which may better improve the detection rate of the primary tumor in HNCUP, are in urgent need.
Cancer-associated fibroblasts (CAFs), accounting for high proportion of most solid tumor mass, play a vital role in tumor growth, migration, and progression (19). The major feature discriminating CAFs from normal fibroblasts is the overexpression of fibroblast activation protein (FAP) (20). The presence of FAP was observed on a variety of epithelial and mesenchymal malignancies (21,22). Recently, 68Ga-radiolabeled fibroblast activation protein inhibitor (FAPI), a novel FAP-targeted PET tracer, has shown great value in the diagnosis of diverse carcinomas (23,24). Furthermore, studies (25,26) have demonstrated that 68Ga-FAPI revealed high uptake in primary tumors and low background noise in the head and neck region. These promising findings indicate that 68Ga-FAPI could serve as a potential alternative to 18F-FDG for the assessment of head and neck cancers.
Thus, the aim of this study was to investigate the value of 68Ga-FAPI PET/CT for identifying the primary tumor of 18F-FDG–negative HNCUP.
MATERIALS AND METHODS
Patient Selection
For patients whose primary tumor could not be identified by thorough medical history, clinical examination, medical imaging (e.g., contrast-enhanced CT, contrast-enhanced MRI, ultrasound, and 18F-FDG PET/CT), and endoscopy, 68Ga-FAPI PET/CT was recommended, based on the decision of a multidisciplinary team in head and neck cancer (Fig. 1A). In addition to patients in whom 18F-FDG findings were negative for localizing the primary tumor, 68Ga-FAPI PET/CT was also recommended to patients in whom 18F-FDG findings were positive for localizing the primary tumor who had undergone a biopsy that resulted in a negative finding. To further investigate the role of 68Ga-FAPI PET/CT in HNCUP, inclusion criteria were as follows: adult patients (age > 18 and < 80 y); pathology-confirmed metastatic cervical carcinoma by fine-needle aspiration; conventional imaging modalities (e.g., contrast-enhanced CT, contrast-enhanced MRI, or ultrasound) could not provide positive finding of primary tumor; both 18F-FDG and 68Ga-FAPI PET/CT were performed. The exclusion criteria were patients with lymphomas or non–head and neck original cancers, confirmed by immunohistochemistry; patients with both positive 18F-FDG and 68Ga-FAPI PET/CT findings for primary tumors, including anaplastic thyroid carcinoma, lymphoepithelioma-like carcinoma, and biopsy-negative but clinically diagnosed nasopharyngeal carcinoma; patients with 2 or more malignant tumors history; and patients unwilling to undergo 18F-FDG or 68Ga-FAPI PET/CT. 18F-FDG PET/CT reported negatively for localization of primary tumor in patients with HNCUP would be regarded as negative 18F-FDG PET/CT findings. This prospective study was approved by Fudan University Shanghai Cancer Center Institutional Review Board (ID 2004216-25) conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards, and all subjects signed an informed consent form.
Flowchart of diagnostic workup (A) and patient selection (B). “YES” means the primary tumor was identified by these techniques and further confirmed by pathology, and “NO” indicates these techniques could not identify the primary tumor. *68Ga-FAPI PET/CT could also identify the primary tumor in these patients.
Radiopharmaceuticals and PET/CT Scanning Procedure
18F-FDG was produced automatically using the Explora FDG4 module with cyclotron (Siemens CTI RDS Eclips ST) in our center. DOTA-FAPI-04 (Jiangsu Huayi Technology Co., Ltd.) was radiolabeled with 68Ga solution (elution from the 68Ge generator IGG100, Eckert & Ziegler) according to the procedure of Lindner et al. (27). The radiochemical purities of 18F-FDG and 68Ga-FAPI were both more than 95%.
18F-FDG PET/CT was performed first, and 68Ga-FAPI PET/CT imaging was then performed within 1 wk. For 18F-FDG PET/CT scanning, patients fasted at least 6 h, maintaining venous blood glucose levels under 10 mmol/L before 18F-FDG administration. This fasting process was not necessary for 68Ga-FAPI PET/CT scanning. After injection of with 260.64 ± 40.81 MBq of 18F-FDG or 143.71 ± 16.19 MBq of 68Ga-FAPI, patients were kept in a quiet environment for approximately 60 min before examination. All images were obtained on a Biograph mCT Flow scanner (Siemens Medical Solutions). PET image datasets were reconstructed iteratively using an ordered-subset expectation maximization iterative reconstruction by applying CT data for attenuation correction. Two experienced nuclear medicine physicians independently analyzed and interpreted the images masked, and they reached a consensus in the case of inconsistency.
Increased radioactivity of primary and metastatic lesions compared with the muscle background uptake was defined as being positive, verified by biopsy or follow-up. For quantitative analysis, maximum or mean of SUV (SUVmax or SUVmean) normalized to body weight was manually computed for primary and metastatic lesions and healthy tissues by drawing a 3-dimensional volume of interest. Meanwhile, the SUVmax ratio for primary tumor was defined as the quotient of the SUVmax of primary tumor and the contralateral normal tissue, and tumor-to-background ratio (TBR) for primary and metastatic lesions was calculated according to the formula TBR = tSUVmax/bSUVmean, where tSUVmax is the SUVmax of the tumor lesion, and bSUVmean is the SUVmean of muscle. The size of primary and metastatic lesions was measured by CT.
Statistical Analysis
All statistical analyses were performed using SPSS 25.0 (IBM). Means with SDs or medians with ranges were used to describe continuous characteristics. To compare the uptake of 18F-FDG and 68Ga-FAPI in metastatic lesions, 2-sample t tests were used. Two-tailed P values less than 0.05 were considered statistically significant.
RESULTS
Patients
A total of 32 patients were enrolled consecutively from our center from June 2020 to February 2021, and 18 patients were included for further analysis according to the inclusion and exclusion criteria (Fig. 1B). The basic clinical characteristics are presented in Table 1. Among the included 18 patients (16 men and 2 women; median age, 55 y; age range, 24–72 y), 2 (11.11%) were infected with Epstein-Barr virus, 6 (33.33%) were infected with human papillomavirus, 16 (88.89%) were pathologically diagnosed with cervical lymph node SCC, and 2 (11.11%) had adenocarcinoma.
Patients Characteristics
Comparison of 18F-FDG and 68Ga-FAPI PET/CT in Metastatic Lesions
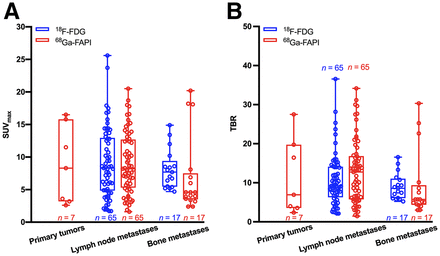
A total of 65 lymph node and 17 bone metastases were detected by both 18F-FDG and 68Ga-FAPI PET/CT (Fig. 2, Table 2, and Supplemental Table 1 [supplemental materials are available at http://jnm.snmjournals.org]). Both tracers showed intensive uptake in lymph node and bone metastases. The mean SUVmax of lymph node metastases was 9.05 ± 5.29 for 18F-FDG and 9.08 ± 4.69 for 68Ga-FAPI (P = 0.975). The TBR for 68Ga-FAPI was a slightly higher than that for 18F-FDG (12.80 ± 8.11 vs. 10.65 ± 6.59, respectively); however, the difference was not significant (P = 0.100). For bone metastases, the mean SUVmax was 8.11 ± 3.00 for 18F-FDG and 6.96 ± 5.87 for 68Ga-FAPI (P = 0.478), and the mean TBR values were 9.08 ± 3.35 and 9.14 ± 8.40 (P = 0.976), respectively. Generally, no significant uptake difference was observed between 18F-FDG and 68Ga-FAPI in lymph node and bone metastases, indicating that 68Ga-FAPI PET/CT had a performance similar to that of 18F-FDG PET/CT in assessing metastases of head and neck cancers.
Box plots of SUVmax (A) and TBR (B) on 18F-FDG versus 68Ga-FAPI PET/CT.
Comparison of Metastatic Lesions on 18F-FDG and 68Ga-FAPI PET/CT in 18 Patients with HNCUP
68Ga-FAPI PET/CT Imaging Results of Primary Tumors
Primary tumors in 7 of 18 (38.89%) patients with 18F-FDG–negative results were identified by 68Ga-FAPI PET/CT and pathologically confirmed by subsequent biopsy. 68Ga-FAPI PET/CT showed a higher detection rate in adenocarcinoma (2/2, 100%) than in SCC (5/16, 31.25%). Primary sites included the nasopharynx (n = 1), palatine tonsil (n = 2) (Fig. 3), submandibular gland (n = 2) (Fig. 4), and hypopharynx (n = 2) (Supplemental Fig. 1 and Table 3).
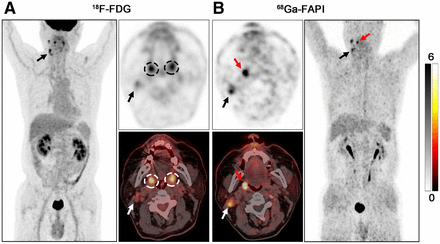
PET/CT scans with 18F-FDG (A) and 68Ga-FAPI (B) in 63-y-old male patient (patient 2) with metastatic SCC of right neck. 18F-FDG PET/CT was negative for detection of primary. Increased uptake of 18F-FDG was detected in palatine tonsils of both right and left sides (A, black and white dashed circles; SUVmax = 6.40 and 6.00, respectively), resulting an SUVmax ratio of 1.07. On 68Ga-FAPI PET/CT, there was asymmetric fullness with intensive uptake in right palatine tonsil (B, red arrow; SUVmax = 8.30), whereas low background uptake was seen in left palatine tonsil (SUVmax ratio = 3.46). Subsequent tonsillectomy confirmed SCC. Black and white arrows indicate metastatic lymph nodes.
PET/CT scans with 18F-FDG (A) and 68Ga-FAPI (B) in a 41-y-old male patient (patient 5) with metastatic adenocarcinoma of right neck. 18F-FDG PET/CT was negative for detection of primary. On 68Ga-FAPI PET/CT, there was intensive uptake in right submandibular gland (B, red arrow; SUVmax = 15.80), whereas low background uptake was seen in left submandibular gland (SUVmax ratio = 6.87). Subsequent surgery confirmed salivary ductal carcinoma. Black and white arrows indicate metastatic lymph nodes.
Primary Tumor Characteristics and Semiquantitative Parameters of 68Ga-FAPI PET/CT
American Joint Committee on Cancer (AJCC) TNM stages for the 7 patients with 18F-FDG–negative results ranged from I to IVC (eighth edition of the AJCC TNM staging system) (28). The smallest primary tumor size detected by 68Ga-FAPI PET/CT was 5 × 3 mm. The mean SUVmax of 68Ga-FAPI for primary tumors was 8.79 (range, 2.60–16.50), and the mean TBR value was 11.50 (range, 2.36–27.50). When compared with the contralateral normal tissue, the primary tumor showed a remarkably higher uptake of 68Ga-FAPI, with a mean SUVmax ratio of 4.50 (range, 2.17–8.21).
DISCUSSION
Identifying the primary tumor remains a concern for patients with HNCUP, though the development in imaging, endoscopy, and pathology techniques has progressed quickly. When no positive findings are obtained using noninvasive procedures, invasive diagnostic procedures such as tonsillectomy are then performed; these invasive procedures have a risk of bleeding or infection (5). Thus, noninvasive methods may be needed for improving the detection rate of primary tumor in HNCUP patients. This study investigated the performance of 68Ga-FAPI PET/CT in identifying the primary tumor of 18F-FDG–negative HNCUP. Our results demonstrated that 68Ga-FAPI can dramatically improve the detection rate of primary tumor in HNCUP patients compared with 18F-FDG. Furthermore, 68Ga-FAPI may show a performance similar to 18F-FDG in assessing metastases.
In the current study, the detection rate of the primary tumor by 68Ga-FAPI PET/CT was 38.89% (7/18). Notably, these patients all exhibited false-negative 18F-FDG PET/CT findings. Sites of false-negative 18F-FDG PET/CT findings included the nasopharynx, palatine tonsil, submandibular gland, and hypopharynx; these sites are different from previously reported observations that the tonsil was the most frequent false-negative location (16). Recently, Serfling et al. (26) reported 68Ga-FAPI PET/CT showed a better visual detection of the malignant primary in Waldeyer’s tonsillar ring than 18F-FDG PET/CT. However, the representative cases could provide positive findings of the primary site by 18F-FDG PET/CT alone in terms of HNCUP. Another study demonstrated that an SUVmax ratio of 18F-FDG uptake between tonsils of ≥1.6 could be regarded as malignancy and used to guide biopsy (29). In this study, 2 patients were diagnosed with palatine tonsil carcinoma by tonsillectomy. Puzzlingly, 18F-FDG PET/CT revealed no visual difference between right and left palatine tonsils in both cases. Furthermore, the SUVmax ratios of 18F-FDG uptake were all approximately equivalent to 1.00 (1.07 and 1.04 for patients 2 and 3, respectively), which was mistaken as physiologic uptake. By contrast, 68Ga-FAPI PET/CT showed intensive uptake in the tumor site and low uptake in the normal site, resulting in a visual difference (SUVmax ratio = 3.46 and 8.21, respectively). In line with our results, Syed et al. (25) demonstrated high 68Ga-FAPI avidity within tumorous lesions and low background uptake in healthy tissues of the head and neck region, again emphasizing the potential role of 68Ga-FAPI PET/CT in detecting palatine tonsil carcinoma, particularly in patients with 18F-FDG–negative results.
In addition to high-grade physiologic uptake in the head and neck, small lesion size was the major reason for the false-negative 18F-FDG findings due to the partial-volume effect and low tumor glucose metabolic activity (30,31). In the current study, 18F-FDG PET/CT missed 3 of 7 primary tumors because of their small size (diameter < 10 mm). Encouragingly, 68Ga-FAPI PET/CT revealed moderate uptake (SUVmax = 2.60, 3.20, and 3.60 for patients 1, 6, and 7, respectively) and a clearly visual difference (SUVmax ratio = 2.17, 2.91, and 3.27, respectively) in these primary tumors with small size, which was consistent with previous research (24). 68Ga-FAPI uptake is primarily based on the expression of FAP on CAFs in a solid tumor microenvironment, and even small T1 stage primary tumors could show a moderate FAP expression (26). Thus, to reduce the false-negative results by 18F-FDG PET/CT, 68Ga-FAPI could serve as an alternative tracer for identifying small primary tumors.
Most of the research focuses on SCC, as it is the most frequent pathologic type of HNCUP (3–5). However, other pathologic types, such as adenocarcinoma and neuroendocrine carcinoma, may cause diagnostic difficulties in clinical practice because of the lack of information regarding these pathologic types. Moreover, for cervical metastatic adenocarcinoma, diagnostic resection of the salivary gland is not recommended even after thorough noninvasive investigations. Furthermore, salivary gland cancers show a paucity of 18F-FDG avidity (32), which was proven again in our study (patients 4 and 5). Several non–18F-FDG radiopharmaceuticals, for example, 18F-fluorothymidine, 68Ga-DOTA-somatostatin analogs, and 18F-fluoromisonidazole, are recommended for detecting the primary tumor of HNCUP (33). However, these tracers are too specific to identify all types of head and neck cancers. Promisingly, recent studies have demonstrated 68Ga-FAPI can evaluate a broad spectrum of malignancies, including adenocarcinoma, neuroendocrine carcinoma, and well-differentiated carcinoma (23,24). In this study, 68Ga-FAPI showed intensive uptake in the submandibular gland (SUVmax = 16.50 and 15.80, respectively), providing sufficient information following surgery. Notably, 68Ga-FAPI had a higher detection rate in adenocarcinoma (2/2, 100%) than SCC (5/16, 31.25%) of HNCUP, indicating that 68Ga-FAPI was more sensitive to adenocarcinoma. However, further research with larger sample sizes is needed to verify this result.
Regarding the detection of regional and distant metastases, the performance of 68Ga-FAPI PET/CT varies among different studies (24,26). In our study, 68Ga-FAPI PET/CT showed a performance (P > 0.05) similar to that of 18F-FDG PET/CT in detecting both lymph node and bone metastases. Because radiation therapy is one of the most important modalities of treating HNCUP, the advantages of 68Ga-FAPI PET/CT in both primary tumors and metastases may play a vital role in gross tumor volume delineation.
There are some limitations in this study. The main limitation is the relatively small number of patients and that the number of pathologic types is imbalanced. In the future, larger population cohort studies with more cancer types need to be considered. Additionally, immunohistochemistry for FAP expression of primary tumors and metastases is lacking. Hence, FAPI imaging and FAP expression control studies are also necessary in the future.
CONCLUSION
This study demonstrated that 68Ga-FAPI PET/CT can improve the detection rate of the primary tumor in HNCUP patients with negative 18F-FDG findings. Furthermore, for evaluating metastatic lesions, 68Ga-FAPI PET/CT showed a performance similar to that of 18F-FDG PET/CT. Because an improved detection rate is necessary in HNCUP, future research on more patients with HNCUP should be considered to evaluate the clinical value of 68Ga-FAPI PET/CT.
DISCLOSURE
This work was funded by National Natural Science Foundation of China (grants 81771861, 81971648, and 81901778) and Shanghai Anticancer Association Program (grant no. HYXH2021004). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does 68Ga-FAPI PET/CT have value for identifying the primary tumor in HNCUP patients with 18F-FDG–negative results?
PERTINET FINDINGS: In this prospective study, 68Ga-FAPI PET/CT improved the detection rate (38.89%) of the primary tumor in HNCUP patients with negative 18F-FDG findings.
IMPLICATIONS FOR PATIENT CARE: Our study provides a new strategy for identifying the primary tumor in patients with HNCUP, which may change their treatment decisions.
ACKNOWLEDGEMENT
We thank the head and neck cancer multidisciplinary team in our center for the great help to our work.
Footnotes
Published online Sep. 30, 2021.
- © 2022 by the Society of Nuclear Medicine and Molecular Imaging.
Immediate Open Access: Creative Commons Attribution 4.0 International License (CC BY) allows users to share and adapt with attribution, excluding materials credited to previous publications. License: https://creativecommons.org/licenses/by/4.0/. Details: http://jnm.snmjournals.org/page/permissions.
REFERENCES
- Received for publication June 28, 2021.
- Revision received September 21, 2021.