Abstract
1311
Objectives: Lutetium-177 prostate specific membrane antigen (177Lu-PSMA) based radioligand therapy (RLT) as salvage/ compassionate therapy in end stage metastatic castration resistant prostate cancer (mCRPC) has reported favourable biochemical and radiological responses. In this study, we aimed to prospectively compare the therapeutic efficacy and safety of 177Lu-PSMA-617 and Docetaxel.
Methods: Chemotherapy-naive patients with mCRPC and high PSMA expressing lesions on 68Ga-PSMA-11 PET/CT were randomly assigned to 177Lu-PSMA-617 (6-7.4 GBq per cycle, every 8 weeks, up to 4 cycles) or Docetaxel (75 mg/m2 per cycle, every 3 weeks, up to 10 cycles). The primary end-point was best PSA response rate defined as the proportion of patients achieving a ≥50% decline in PSA from baseline. Secondary end-points included radiological response, molecular response, progression-free survival (PFS) and safety profile.
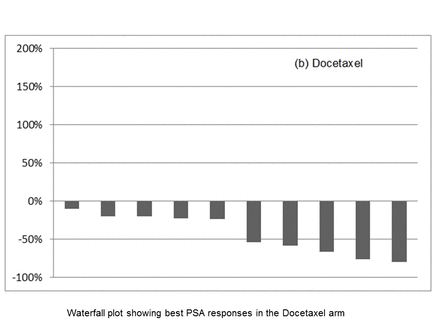
Results: Twenty men with mCRPC, who had not received prior chemotherapy, were randomized to receive 177Lu-PSMA-617 or Docetaxel. Ten patients received a median cumulative dose of 15 GBq (range 6-24 GBq) of 177Lu-PSMA-617 over 1-4 cycles at intervals of 8-16 weeks while another ten patients were administered 5-10 cycles of Docetaxel at a dose of 75 mg/m2 every 3 weeks. The primary end point, i.e. the best PSA response was achieved in 6/10 (60%, 95% CI: 31.3%-83.2%) patients in the 177Lu-PSMA arm compared to 5/10 (50%, 95% CI: 23.7%-76.3%) patients in the Docetaxel arm (p=1.00). According to RECIST 1.1, the best objective response rate was 60% (95% CI: 23.1%-88.2%) in the 177Lu-PSMA arm versus 40% (95% CI: 16.8%-68.7%) in the Docetaxel arm (p=0.61). Further, as per the adapted PERCIST 1.0, the best molecular response rates, in the 177Lu-PSMA and Docetaxel arms, were 50% (95% CI: 18.8%-81.2%) and 40% (95% CI: 16.8%-68.7%) respectively (p=1.00). The median PFS in the 177Lu-PSMA and Docetaxel arms were 3.5 months and 4.0 months respectively (p=0.60). Of the treatment-related adverse events, grade 3 diarrhoea, palmar-plantar erythrodysesthesia syndrome, dyspnea, anaemia and febrile neutropenia were observed in one (10%) patient each in the Docetaxel arm with two (20%) patients requiring dose interruption/ reduction. In contrast, one patient (10%) in the 177Lu-PSMA arm experienced grade 4 thrombocytopenia.
Conclusions: With its relatively high safety margin and comparable therapeutic efficacy to that of Docetaxel, 177Lu-PSMA has a definite role in the management of mCRPC and could also be potentially employed in the first-line or second-line setting, rather than being solely reserved for advanced end-stage disease after exhausting all other treatment options.