Terbium-161 is a β-emitting radionuclide that resembles 177Lu in terms of its in vivo and in vitro chemical and pharmacokinetic properties, exhibiting similar behavior with regard to radioligand-specific cell uptake and internalization, as well as emitting a modest fraction of photons useful for posttherapy imaging. Unlike 177Lu, a significant amount of conversion and Auger electrons are emitted per decay, making it particularly appealing for targeted radionuclide therapy (1).
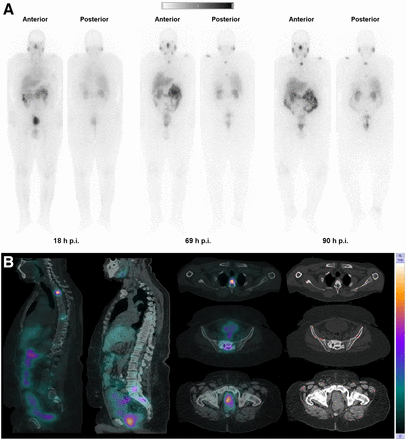
Here, we present whole-body scintigraphic and SPECT/CT images acquired with 161Tb-PSMA-617 in a 69-y-old man diagnosed with metastatic prostate cancer refractory to hormonal therapy and chemotherapy who was referred for PSMA radioligand therapy (Fig. 1).
(A) Whole-body images at different time points after injection. (B) Representative SPECT/CT sagittal and axial slices and CT axial slices demonstrating physiologic biodistribution of 161Tb-PSMA in lacrimal, parotid, and submandibular glands; nasopharyngeal mucosa; liver; intestinal tract; kidneys; and urinary bladder, as well as pathologic uptake in primary prostate tumor and metastatic bone lesions. p.i. = after injection.
The patient received an empiric well-tolerated dose of 161Tb-PSMA-617 (5,550 MBq) without having acute or early adverse events (compassionate use on a named-patient basis under the local regulatory framework and international ethical and radiation safety standards).
Two γ-energies with high frequencies were identified from the decay scheme of 161Tb: 48.9 keV with a 17% frequency and 74.5 keV with a 10.2% frequency (1). As a result, whole-body planar and SPECT/CT scanning protocols have been created. Spatiotemporal distribution of the radionuclide in the target and nontarget potentially dose-limiting organs was obtained by acquiring time-sequential planar and SPECT/CT datasets: 18 h after injection, 69 h after injection, and 90 h after injection. SPECT/CT images were acquired from the lower cervical level to the pelvis at 69 h after injection, aiding in more refined image-derived activity quantification and characterization of tissue kinetics. The obtained images were of good quality, enabling visualization of all previously identified PSMA-avid primary and metastatic bone lesions using a 68Ga-PSMA PET/CT scan.
In-human posttherapy imaging with 161Tb SPECT/CT has been proposed as a predefined clinical protocol using a radiolabeled somatostatin analog of up to 113 h after injection (2). We present here, to the best of our knowledge, the first-in-humans posttherapy 161Tb-PSMA SPECT/CT imaging.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Feb. 9, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
- Received for publication December 5, 2022.
- Revision received January 31, 2023.