Abstract
With integrated whole-body PET/MRI, a novel metabolic–anatomic imaging technique recently has been introduced into clinical practice. This review addresses PET/MRI of bone tumors, soft-tissue sarcoma, melanoma, and lymphoma. If PET/MRI literature is not yet available for some types of tumors, potential indications are based on available PET/CT and MRI data. PET/MRI seems to be of benefit in T-staging of primary bone tumors and soft-tissue sarcomas. With regard to N-staging, PET/MRI can be considered similarly accurate to PET/CT when applied as a whole-body staging approach. M-staging will benefit from MRI accuracy in the brain, the liver, and bone.
Imaging plays a key role in diagnosis and staging in oncology. Evaluation of local tumor extent and detection of potential locoregional lymph node or distant metastases according to the periodically revised standardized TNM cancer staging system (1) directly affects patient care by defining the most suitable therapy. With integrated whole-body PET/MRI, a new metabolic–anatomic imaging modality has been introduced into clinical practice. Despite the fact that the solution of basic problems, such as adequate MRI-based attenuation correction, is still a work in progress (2), reports on initial clinical experiences with PET/MRI in oncology are already available. In this second part of our review on PET/MRI in oncologic applications, we summarize the available first experiences with PET/MRI in bone tumors, soft-tissue sarcoma, melanoma, and lymphoma. In fields where PET/MRI data are lacking, we outline the potential role of PET/MRI on the basis of the PET/CT and MRI literature. To provide further information on PET/MRI, we refer to our own unpublished experiences with PET/MRI in parts of this article. This contribution needs to be understood as supported solely by the authors’ experience and should not be misinterpreted as evidence-based knowledge. In general, PET/MRI will be indicated and perform superiorly to PET/CT in those oncologic indications that require high soft-tissue contrast for diagnosis. Indications in which soft-tissue contrast is of limited importance will probably remain the domain of the workhorse, PET/CT (Table 1).
Indications in Which PET/MRI Is Favorable to PET/CT, Depending on Tumor Entity
TUMORS OF THE BONE
Primary Bone Tumors
Initial Diagnosis and T-Staging
Aside from conventional radiography, MRI is the preferred imaging modality for the diagnosis and T-staging of malignant primary bone tumors (e.g., osteosarcomas) (3). An overlap of imaging findings between benign and malignant masses and the diagnosis of malignancy are typically aided by advanced MRI techniques. Nuclear MR spectroscopy, for example, has been shown to enhance the discrimination of benign from malignant tumors (4). The diagnostic accuracy reported for MRI in T-staging was 94% in a study retrospectively evaluating a heterogeneous cohort consisting of patients with bone and soft-tissue sarcoma (5). In the same study, the combined metabolic–anatomic approach using 18F-FDG PET/CT for T-staging, with 96% accuracy, was reported to exceed even the excellent performance of MRI, a result that may be considered rather unexpected in view of the inferior soft-tissue contrast of CT to that of MRI and the well-known limitations of 18F-FDG PET for T-staging of various tumors. However, data on integrated PET/MRI for T-staging of malignant primary bone tumors are not yet available. We believe that PET/MRI may not increase the diagnostic accuracy of T-staging of bone tumors over MRI alone, but a whole-body PET/MRI approach may offer TNM staging with high accuracy in a single session. T-staging will benefit from the MRI data, N-staging will benefit from PET, and M-staging will benefit from the combination. The PET component of PET/MRI will be able to guide diagnostic biopsies and help maximize the accuracy of correct staging and grading, with a consequent impact on treatment and outcome (3).
N-Staging
Another advantage of integrated PET/MRI in malignant primary bone tumors is that accurate local staging with high resolution can be paired with a sensitive metabolic whole-body staging examination. In a retrospective study on 117 patients, 18F-FDG PET/CT had a sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of 88%, 97%, 82%, 98%, and 96%, respectively, and was significantly more accurate for N-staging in malignant bone tumors than conventional staging examinations (MRI of the tumor location and whole-body CT) (5). Analogously, PET/MRI is expected to be of similar accuracy to PET/CT for the detection of lymph node metastases from malignant bone tumors. Direct comparison to conventional morphologic imaging reveals that 18F-FDG PET foremost contributes to the sensitivity of lymph node metastasis detection (53% vs. 72%) at a high level of specificity (5).
Restaging and Response to Therapy
Patients with relapsing malignant bone tumors have a poor prognosis (3). Although there is no guideline-established role for metabolic imaging in restaging of bone tumors, some promising results on the diagnostic value of 18F-FDG PET/CT can be found in the literature. 18F-FDG PET/CT has high accuracy in restaging, with a sensitivity, specificity, and accuracy of 87%, 97%, and 94%, respectively (6). In a prospective comparative study assessing therapy response in pediatric osteosarcomas, 18F-FDG PET was able to discriminate responders from nonresponders, but CT and MRI (measurements of the tumor volume) were not (7). Interestingly, a subgroup analysis of the same study revealed that 18F-FDG PET was not beneficial for therapy response evaluation in Ewing sarcoma–type tumors. MRI techniques that go beyond tumor size and volume, such as dynamic contrast-enhanced MRI and diffusion-weighted MRI, have proven sensitive to chemotherapy-induced tumor necrosis and thus can also be used to evaluate therapy response (8). However, both MRI and 18F-FDG PET are of value for restaging and response assessment in primary bone tumors. The integration of these components by PET/MRI offers a whole new field for research on this topic.
Bone Metastases
Approximately 50% of cancer patients are estimated to experience severe bone pain. Frequently, especially in prostate, breast, and lung cancer, metastases are found to be causal (9). Imaging plays a pivotal role in the diagnosis of a metastatic osseous spread, and morphologic (CT and MRI) and functional (scintigraphy and PET) imaging modalities are routinely applied in cancer patients. A metaanalysis comparing the diagnostic performance of radiologic and nuclear medicine methods, and their combination, for bone metastasis detection in more than 15,000 patients provided the broadest data so far (10). 18F-FDG PET, with a sensitivity of 90% and specificity of 97%, was as accurate as MRI, at 91% and 95%, respectively. When integrated 18F-FDG PET/CT was applied, sensitivity increased to 94%, and the same high specificity of 97% was preserved (10). A subanalysis revealed a higher sensitivity (95%) alongside a lower specificity (89%) when studies that included diffusion-weighted MRI sequences were compared with studies that did not (88% and 97%, respectively). 18F-FDG PET, 18F-FDG PET/CT, and MRI were significantly more accurate for bone metastasis detection than were bone scintigraphy and stand-alone CT (10). A smaller but prospectively designed comparative study reported a higher overall accuracy for whole-body MRI (91%) than for 18F-FDG PET/CT (78%). Whole-body MRI, according to that study, was clearly superior to 18F-FDG PET/CT in sensitivity. Sensitivity was 94% for MRI and 78% for 18F-FDG PET/CT, with comparable specificities (76% and 80%, respectively) (11). One reason for the significantly better performance of MRI in that study may have been a larger number of patients with diffuse (but small-volume) osseous metastases, which may be missed on 18F-FDG PET and on CT. The smallest detectable bone metastasis was 2 mm on MRI, compared with 5 mm on 18F-FDG PET/CT (11). The results of these studies are well suited to giving a general impression on the diagnostic performance of different imaging methods. On the other hand, reliable data on diagnostic accuracy in certain cancer types are of higher practical relevance. For example, whole-body MRI was less sensitive (77% vs. 92%) and specific (92% vs. 98%) than 18F-FDG PET/18F-FDG PET/CT for the detection of bone metastases, as well as being less sensitive (77% vs. 86%) but more specific (92% vs. 88%) than bone scintigraphy in lung cancer patients (12). In contrast, MRI was more sensitive than 18F-FDG PET and bone scintigraphy (97% vs. 83% vs. 87%) for the detection of bone metastases in breast cancer patients (13). These findings indicate that for a reasonable judgment on the favored imaging modality in a certain cancer entity, differentiated studies are indispensible. Both PET/CT and MRI suffer from false-negative results requiring periodic restaging (14).
The presented data outline the high capability of 18F-FDG PET for assessing the skeletal system for metastases and the additional value of integrated PET in combination with a coregistered morphologic reference, that is, 18F-FDG PET/CT. Metabolic imaging with PET is clearly advantageous over bone scintigraphy and stand-alone CT. MRI, especially when incorporating diffusion-weighted sequences, seems to have an at least comparable accuracy to 18F-FDG PET/CT. MRI can depict metastatic bone marrow invasion before the development of structural damage visible on CT or of responsive osteoblastic activity visible on bone scintigraphy (15). Moreover, diffusion-weighted MRI is of use in assessing the response of bone metastases to systemic therapy, as has been shown for osseous prostate cancer metastases treated with antiandrogens (16). For integrated skeletal imaging in combination with PET, MRI is, therefore, more promising than CT. PET/MRI will enhance the detection of bone metastases and is strongly awaited as an imaging method that will exceed the performance of PET/CT (Fig. 1).
A 55-y-old patient with metastatic breast carcinoma. Unremarkable sclerotic lesion (arrow) of left femoral bone on axial CT (A) was diagnosed as metastasis only because it was strongly 18F-FDG–avid on PET/CT (B). Whole-body MRI using T1-weighted fat-suppressed sequence (C) clearly depicts metastasis as contrast-enhancing mass (arrowhead). Corresponding PET/MR image (D) demonstrates precise metabolic–anatomic correlation of this technique for bone imaging.
SOFT-TISSUE SARCOMA
Initial Diagnosis and T-Staging
In cases of an undetermined soft-tissue mass, MRI with its high soft-tissue contrast and lack of radiation exposure is often the first choice in cross-sectional imaging (17), especially in children. In this setting, differentiation of malignant from benign masses is of utmost importance. Reports of up to 90% accuracy for MRI in discriminating benign from malignant lesions (18) are hampered by the results of other MRI studies revealing that a correct classification in accordance with histopathology can be reached in only 25%–33% of cases (19). Reports are emerging on the utility of advanced MRI techniques, such as nuclear MR spectroscopy, that could potentially contribute to differentiation between malignant and benign soft-tissue masses (20), but for a conclusive and secure diagnosis, patients with indecisive MRI or CT findings will undergo biopsy in most cases (17). The availability of additional PET data will not obviate definite histopathologic diagnosis by biopsy; thus, PET/MRI is not expected to be of additional use in the primary diagnosis of undetermined soft-tissue masses. But analogously to 18F-FDG PET, there might be an indication for integrated whole-body PET/MRI in the diagnostic algorithm in cases of sarcoma in which the primary location is unknown (21).
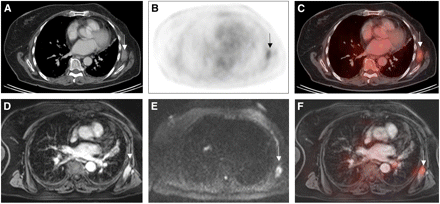
Based on its high soft-tissue contrast, MRI is the modality of choice for assessing local tumor infiltration (22). 18F-FDG PET does not add information to MRI for T-staging in soft-tissue sarcomas (23). On the other hand, 18F-FDG PET provides additional prognostic information. Higher metabolic activity (maximum standardized uptake value) of the primary tumor has recently been shown to predict shorter survival in a retrospective evaluation of 41 children with rhabdomyosarcoma (24). Planning of surgical tumor excisions is often based on the depth of infiltration found on MRI. 18F-FDG PET can determine a safe tumor-free surgical margin by applying a threshold of less than 1 for maximum standardized uptake value (25). PET/MRI may thus be used to guide the surgeon in preoperative planning of tumor resection. Our own initial experience with PET/MRI in patients with soft-tissue sarcoma indicates that integrated PET/MRI can replace stand-alone morphologic imaging, with a focus of the 18F-FDG PET component for prognostic questions and assessment of N-stage and M-stage (Fig. 2).
A 63-y-old patient with sarcoma metastasis in left anterior serratus muscle. Axial contrast-enhanced CT (A) shows ill-defined muscle-isodense soft-tissue mass (arrow) that is 18F-FDG–avid on 18F-FDG PET (B) and 18F-FDG PET/CT (C). Extent of this mass is better depicted on axial contrast-enhanced fat-suppressed MRI (D). Diffusion-weighted MR image (E) depicts this metastasis as high-signal diffusion-restricted lesion (arrow) and demonstrates sensitivity of diffusion-weighted MRI. 18F-FDG PET/MR image is also shown (F).
N-Staging
On the basis of experience with 18F-FDG PET versus conventional morphologic imaging, PET/MRI is promising for N-staging of patients with soft-tissue sarcoma. A recent prospective comparative study on soft-tissue sarcoma staging reported that 18F-FDG PET is superior to conventional diagnostic imaging techniques (MRI of the tumor primary site, whole-body CT, and 99mTc-methyl diphosphonate bone scintigraphy) for N-staging, with a sensitivity of 95% versus 25%, respectively (26). 18F-FDG PET/CT was reported to change the N-stage in 4 of 13 rhabdomyosarcoma patients in a smaller series (21). Interestingly, a retrospective study on PET with the radiotracer 11C-choline reported 100% accuracy for N-staging in soft-tissue sarcomas and an overall accuracy of 94% for TNM staging, versus 50% for conventional imaging modalities (27). The presence of highly metabolically active lymph node metastases is predictive of a shorter survival, and thus 18F-FDG PET for N-staging provides relevant prognostic information (24). The indication for PET/MRI will thus include combined T-, N-, and M-staging in a single session.
Restaging and Response to Therapy
MRI, whole-body CT, and bone scintigraphy or a combination of these methods is applied for restaging of patients with soft-tissue sarcoma. A metaanalysis on the diagnostic performance of 18F-FDG PET/CT reported 100% sensitivity, 96% specificity, 99% PPV, 100% NPV, and 99% overall accuracy for restaging sarcoma patients (28). In the same analysis, 18F-FDG PET was more accurate than stand-alone CT. Comparative studies have proven that a side-by-side reading of 18F-FDG PET and conventional imaging techniques leads to a significantly higher number of correct therapeutic decisions than does conventional imaging alone (91% vs. 59%) (26). There is evidence that the simple evaluation of tumor size is inadequate for assessing the response of soft-tissue sarcomas to therapy (29), and thus markers of tumor biology and metabolism are of increasing importance. Conventional MRI, as well as functional MRI, provides a whole bouquet of information on tumor size, perfusion, tissue composition, and extent of tumor necrosis and plays an important role in assessing the response of soft-tissue sarcomas (30). Diffusion-weighted MRI, for example, has been shown to provide a measure of tumor cellularity, with a reverse linear relation between measured adenylate cyclase values and cellular count in soft-tissue sarcoma histopathology (31). Moreover, a strong correlation between a change in tumor adenylate cyclase and a change in tumor volume under chemotherapy has been demonstrated (32). Diffusion-weighted MRI thus provides a valuable tool for the assessment of cytotoxic therapy response. Metabolic imaging with 18F-FDG PET is also sensitive for assessing the therapeutic response of patients with soft-tissue sarcoma. In a prospective study, the reduction of metabolic activity between 2 consecutive 18F-FDG PET examinations during chemotherapy correctly predicted pathologic tumor response in 95% of patients (n = 42) and was more accurate for this prediction than was a reduction of tumor size (33). With a threshold of a 60% decrease in maximum standardized uptake value, 18F-FDG PET had 100% sensitivity and 71% specificity for the prediction of pathologic tumor response (33). A different study, applying a threshold of a 35% decrease in maximum standardized uptake value under chemotherapy, discriminated therapy responders from nonresponders with 100% sensitivity and 67% specificity as early as after the first chemotherapy cycle (34). Despite these promising reports, recent emerging evidence has questioned the value of 18F-FDG PET for the evaluation of neoadjuvant therapy in soft-tissue sarcoma (35), indicating that there still is research to be done on this topic. Follow-up data on the diagnostic performance of integrated PET/MRI are currently not available. The simultaneous acquisition of dynamic functional MRI and PET information—for example, simultaneous contrast-enhanced dynamic MRI and dynamic PET—offers a new quality of bioinformation in soft-tissue sarcoma restaging and therapy response assessment.
MELANOMA
Initial Diagnosis and T-Staging
Diagnosis of malignant melanoma of the skin is based on clinical inspection and full-thickness biopsy, and tumor stage according to the American Joint Committee on Cancer (AJCC) classification is based on the presence of ulceration and tumor thickness as confirmed by histopathology (36). Generally, CT, MRI, and 18F-FDG PET depict melanomas with variable sensitivity and specificity depending on size and location. However, there is no current indication for imaging in the primary diagnosis and T-staging of malignant melanomas.
N-Staging
The risk for locoregional metastases is stratified according to the depth of tumor infiltration (AJCC classification). According to current guidelines, in melanoma patients with a tumor thicker than 1 mm, sentinel lymph biopsy should be considered, followed by locoregional lymphadenectomy in cases of positivity for metastatic disease (37). This algorithm is based on the weak performance of imaging modalities for N-staging in malignant melanoma when small metastases and micrometastases are considered. A metaanalysis on the diagnostic performance of different imaging modalities for N-staging in melanoma patients reported a sensitivity and specificity of 60% and 97% for ultrasonography, which was the highest accuracy achieved in comparison with the low values for CT (9% and 92%, respectively), 18F-FDG PET (30% and 96%, respectively), and 18F-FDG PET/CT (11% and 97%, respectively) (38). The prospectively evaluated sensitivity, specificity, PPV, and NPV of sentinel lymph node biopsy for detection of occult locoregional lymph node metastases was 94%, 100%, 100%, and 99%, respectively (39). The explanation for the low sensitivity of imaging studies can potentially be found in the small mean tumor volume of lymph node metastases (<5 mm3) that is regularly found in melanoma patients (39,40). These small nests of tumor cells are frequently missed on morphologic and metabolic scans. However, in patients with positive results on sentinel lymph node biopsy, imaging is required and recommended to exclude further metastatic spread (37). In a prospective study on 18F-FDG PET in AJCC stage III patients with positive sentinel lymph node biopsy and with CT, MRI, and ultrasonography negative for disseminated disease, 18F-FDG PET revealed unknown distant metastases and thus upstaged 4 (12%) of 33 patients to AJCC stage IV (41). Whole-body MRI, with a sensitivity, specificity, PPV, NPV, and accuracy of 66%, 77%, 84%, 55%, and 67%, respectively, for the detection of lymph node metastases, has been shown to be equal in accuracy to whole-body CT (42) and inferior to 18F-FDG PET/CT (43). Another comparative study on the same topic found whole-body MRI to be at least as accurate as 18F-FDG PET/CT for N-staging, using a combination of conventional MRI sequences and diffusion-weighted MRI (44). The rather sobering performance of whole-body MRI for N-staging in malignant melanoma has to be seen in the context of major advantages that whole-body MRI provides in the detection of subcutaneous, bone, liver, and brain metastases. Integrated PET/MRI will, therefore, be a tool to achieve whole-body melanoma staging in a single session (Fig. 3).
A 65-y-old patient with pathologically confirmed T4b malignant melanoma of right leg. Coronal whole-body 18F-FDG PET/CT (A), axial contrast-enhanced CT (B), axial 18F-FDG PET (C), and axial 18F-FDG PET/CT (D) show multiple enlarged and 18F-FDG–avid inguinal and iliac lymph node metastases (arrows). Coronal 18F-FDG PET/MRI (E), axial T1-weighted MRI (F), axial 18F-FDG PET performed simultaneously with MRI, (G) and axial 18F-FDG PET/MRI (H) are as accurate as PET/CT for visualization of lymph node metastases.
Restaging and Response to Therapy
The risk of tumor recurrence in malignant melanoma depends on the primary-tumor thickness. In low-risk patients (tumor thickness < 1 mm, AJCC stages 0–Ib), surveillance relies solely on clinical follow-up and routine self-examinations of the skin and lymph nodes (37). Restaging with ultrasound, CT, MRI, and PET/CT according to current guidelines (37) is reserved to, but not mandatory in, high-risk patients. A retrospective study in high-risk melanoma patients found that 18F-FDG PET/CT, with a sensitivity, specificity, NPV, and PPV of 97%, was more accurate for the detection of tumor recurrence than the tumor marker S100 (respective values: 86%, 45%, 61%, and 76%) (45). The same study revealed that PET/CT, compared with S100, had a significantly higher prognostic value for cancer-related mortality. In a different study, S100 positivity was used as a pretest for tumor recurrence detection in asymptomatic high-risk melanoma patients, with a sensitivity of 100%, a specificity of 90%, a PPV of 96%, and an NPV of 100% (46). According to the results of a recent metaanalysis, the PPV of PET/CT for the detection of recurrent metastases is stage-dependent, yielding a higher PPV in high-risk patients (80%) than in intermediate-risk patients (63%) and low-risk patients (33%) (38). Besides the reliable detection, a precise localization of relapsing disease is crucial for therapeutic, that is, surgical, decisions. A prospective study on the impact of imaging on surgical decision making in melanoma patients reported that in 25%–75% of patients, surgery was adapted on the basis of findings on 18F-FDG PET/CT (47). MRI has also been shown to influence the therapeutic approach in melanoma patients. Therapy was changed in 64% of patients (n = 41) and included adaption of the surgical approach in 10 patients after reevaluation of disease spread on whole-body MRI, including diffusion-weighted imaging (43). Moreover MRI, with its higher soft-tissue contrast, has proven to be clearly superior for the detection of metastases in important organ sites, with an impact on therapy strategies for, for example, liver and brain (42–44). Both 18F-FDG PET/CT and MRI are sensitive to changes under local and systemic therapy. 18F-FDG PET/CT has been shown to outperform the tumor marker S100 in the discrimination of responders from nonresponders in a study on stage IV melanoma patients (48). Responders to chemotherapy identified by 18F-FDG PET/CT in a retrospectively performed evaluation have been proven to have a longer progression-free and overall survival than nonresponders (48). Experimental studies performed mainly ex vivo on melanoma xenografts reported that the amount of tumor cell necrosis and the oxygenation status of tumor cells could be assessed using nuclear MR spectroscopy (49). More clinically relevant is the potential of MRI in restaging brain metastases, as these have been proven to be predictive of a poor prognosis (48). Data on the performance of integrated PET/MRI in melanoma restaging and response assessment are awaited but not available at present. We expect clinical PET/MRI to become a 1-stop-shop whole-body N- and M-staging tool in high-risk patients (AJCC stages > III) (Fig. 3).
LYMPHOMA
Initial Diagnosis and Staging
In both Hodgkin disease and non-Hodgkin lymphoma, imaging plays an important role for primary diagnosis and staging, with an impact on therapy (50). Current guidelines encourage the use of 18F-FDG PET/CT for primary staging of 18F-FDG–avid and potentially curable lymphomas (e.g., diffuse large B-cell lymphoma, Hodgkin disease), particularly with regard to the 2007 revised response criteria (51,52) that include the evaluation of tumor tissue metabolism (53). The performance of 18F-FDG PET in lymphoma has been assessed extensively in the literature. In a retrospective analysis, 18F-FDG PET/CT, with an accuracy of 94%, was more accurate than conventional staging procedures (89%), including CT and bone marrow biopsy (54). Some authors argue that 18F-FDG PET/CT might even eliminate the need for bone marrow biopsy in the primary staging of Hodgkin disease because 18F-FDG PET/CT is highly sensitive and specific for bone marrow involvement in this disease, at 92% and 90%, respectively (55). Except for cerebral lymphoma, whole-body MRI is currently of high research interest but minor importance in the staging algorithm of lymphoma patients. This situation soon might substantially change, and whole-body MRI might become a good alternative to CT (56). There is evidence that whole-body diffusion-weighted MRI, with a sensitivity of 90% and a specificity of 94%, could be as accurate as 18F-FDG PET/CT for the staging of Hodgkin disease and non-Hodgkin lymphoma (57,58). Interestingly, particularly in small, indolent lymphomas that are not considerably 18F-FDG–avid, additional information might be gained from diffusion-weighted MRI (59). MRI, in a recent metaanalysis, was almost equally sensitive (90%) to 18F-FDG PET/CT for the detection of bone marrow involvement in Hodgkin disease but was less specific (75%) (55). The addition of diffusion-weighted MRI significantly increased the accuracy of whole-body MRI for primary staging of lymphoma patients, leading to 94% concordance with the findings on 18F-FDG PET/CT (58). Although not leading to an underestimation of disease, staging with whole-body MRI was found in one report to bear the risk of considerably overstaging lymphoma patients, with a consequent impact on therapeutic decisions (60). This more critical report outlines the need for more reliable data on the utility of MRI for whole-body staging of lymphoma patients. However, in lymphoma patients our first experiences with whole-body PET/MRI, which we currently acquire in addition to 18F-FDG PET/CT, indicate a good concordance with findings on PET/CT (Fig. 4). Because of the lack of radiation exposure of MRI, compared with CT, PET/MRI may evolve as an alternative to PET/CT in potentially curable patients.
A 49-y-old patient with non-Hodgkin lymphoma. Bone-windowed coronal CT (A) does not depict spread of paravertebral lymphoma masses (arrowhead) into twelfth thoracic and first lumbar vertebrae, which are visible on coronal 18F-FDG PET/CT (arrows) (B). Coronal short-τ inversion recovery MRI (C) and coronal 18F-FDG PET/MRI (D) better depict and confirm bone infiltration (arrows).
Restaging and Response to Therapy
Data on restaging lymphoma patients and assessing their response to therapy are not available at present. Generally, the therapy regime initiated in lymphoma patients depends on the stage of the disease and then is adapted to the individual response and risk of progression (50). Different studies have assessed the option of monitoring therapy response early so as to adapt tumor treatment to therapy response (61). Although this approach is still considered experimental, all other patients will be restaged after completion of therapy. In recent years, 18F-FDG PET and 18F-FDG PET/CT have been included in the guidelines and are considered essential for therapy monitoring of diffuse large B-cell lymphoma and Hodgkin disease (51). Metaanalyses have demonstrated that 18F-FDG PET and 18F-FDG PET/CT are capable of assessing response early and have detected residual disease with up to a 90% sensitivity and 91% specificity in patients undergoing classic chemotherapy (62,63). These imaging methods are predictive of progression-free survival, with negative 18F-FDG PET findings indicating a higher probability of progression-free survival (64,65). These promising results are hampered by the results of more recent prospective studies, which were undertaken to evaluate the predictive value of 18F-FDG PET for early evaluation of therapy evaluation but found a rather low PPV for disease progression (66,67). 18F-FDG PET/CT findings after 2–3 cycles had a low PPV of 42% for progression or relapse of disease and a NPV of 77% (67). This low PPV has been reported in studies evaluating therapy response to rituximab-based chemotherapy (rituximab, cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone [R-CHOP]). The available literature indicates that false-positives are more frequently seen in rituximab-based therapy schemes than in classic chemotherapy (66). Moreover, for various reasons, false-positive as well as false-negative PET results occur (68). However, the PPV and NPV of 18F-FDG PET/CT for progression-free survival after completion of chemotherapy were 71% and 80%, respectively, even for R-CHOP (67). With regard to these problems, MRI, especially diffusion-weighted imaging, might be of value in restaging of disease and evaluation of therapy response. MRI, by applying a combination of morphologic (size) and functional (adenylate cyclase measurements) parameters, has been shown to detect 100% of residual lymph node sites that were positive on 18F-FDG PET/CT (reference standard) but resulted in 2 false-positive lesions in a comparative prospective pilot study that included 15 patients receiving chemotherapy (69). According to a subanalysis of the same study, the evaluation of morphologic MRI sequences led to a high number of false-positive lesions. The combination of size criteria with visual ADC analysis then reduced the number of false-positive findings to 2 lesions. Lymph nodes with residual disease, as well as false-positive lymph nodes, demonstrated a significant increase in adenylate cyclase compared with the baseline scan, indicating that the diagnosis on diffusion-weighted MRI was correct (69). The use of PET/MRI instead of PET/CT in the surveillance of lymphoma patients undergoing chemotherapy may also be helpful for the differentiation of thymic rebound from recurrent lymphoma of the mediastinum. Chemical shift MRI provides MR images with 2 different contrasts (in-phase and opposed-phase images), depending on the fat-to-water ratio. This technique, by the detection of fat within a tissue mass, allows for the discrimination of hyperplastic thymic tissue from tumor tissue (70).
Although these potential advantages need to be confirmed by larger studies, functional PET/MRI comprising diffusion-weighted and chemical shift imaging represents a promising tool for response assessment in lymphoma patients.
CONCLUSION
Literature on integrated PET/MRI in oncologic applications is still limited. According to first experiences with this imaging technique and the available data on whole-body MRI and PET/CT, PET/MRI can be expected to be of benefit in T-staging of primary bone tumors and soft-tissue sarcomas. For N-staging, PET/MRI seems to provide similar accuracy to PET/CT. The diagnostic performance of the different imaging modalities for M-staging strongly depends on the location—that is, the organ harboring metastases. Therefore M-staging will benefit from MRI soft-tissue contrast and accuracy in the brain, the liver, and bone. The simultaneous acquisition of functional MRI and PET data promises to enhance assessments of tumor response to therapy. In general, PET/MRI will be indicated and perform superiorly to PET/CT in those oncologic indications that require high soft-tissue contrast for diagnosis. Indications in which soft-tissue contrast is of limited importance will probably remain the domain of the workhorse, PET/CT.
Footnotes
Published online Jul. 10, 2012.
Learning Objectives: On successful completion of this activity, participants should be able to describe (1) the advantages and disadvantages of PET/MRI in oncologic applications in comparison to conventional imaging methods and PET/CT; (2) the limitations of PET/MRI compared with invasive staging procedures (biopsy); and (3) the metabolic–anatomic imaging procedure of choice (PET/MRI vs. PET/CT) based on tumor entity and location.
Financial Disclosure: The authors of this article have indicated no relevant relationships that could be perceived as a real or apparent conflict of interest.
CME Credit: SNM is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNM designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credit. Physicians should claim only credit commensurate with the extent of their participation in the activity.
For CE credit, participants can access this activity through the SNM Web site (http://www.snm.org/ce_online) through August 2013.
- © 2012 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication May 24, 2012.
- Accepted for publication June 26, 2012.