Abstract
Our objective was to prospectively explore the diagnostic value of 18F-FDG PET/CT for preoperative staging in endometrial carcinomas and to investigate whether 18F-FDG PET–specific quantitative tumor parameters reflect clinical and histologic characteristics. Methods: Preoperative 18F-FDG PET/CT was prospectively performed on 129 consecutive endometrial carcinoma patients. Two physicians who did not know the clinical findings or staging results independently reviewed the images, assessing primary tumor, cervical stroma involvement and metastatic spread, and determining maximum and mean standardized uptake value (SUVmax and SUVmean, respectively) for tumor, metabolic tumor volume (MTV), and total lesion glycolysis (TLG). All parameters were analyzed in relation to histomorphologic and clinical tumor characteristics. Receiver-operating-characteristic curves for identification of deep myometrial invasion and lymph node metastases were generated, and MTV cutoffs for predicting deep myometrial invasion and lymph node metastases were calculated. Results: The sensitivity, specificity, and accuracy of 18F-FDG PET/CT for the detection of lymph node metastases were 77%–85%, 91%–96%, and 89%–93%, respectively. SUVmax, SUVmean, MTV, and TLG were significantly related to deep myometrial invasion, presence of lymph node metastases, and high histologic grade (P < 0.015 for all) and independently predicted deep myometrial invasion (P < 0.015) and lymph node metastases (P < 0.025) after adjustment for preoperative histologic risk (based on subtype and grade) in endometrial biopsies. Optimal cutoffs for MTV in predicting deep myometrial invasion (20 mL) and the presence of lymph node metastases (30 mL) yielded odds ratios of 7.8 (P < 0.001) and 16.5 (P = 0.001), respectively. Conclusion: 18F-FDG PET/CT represents a clinically valuable tool for preoperatively evaluating the presence of lymph node metastases in endometrial carcinoma patients. Applying MTV cutoffs for the prediction of deep myometrial invasion and lymph node metastases may increase diagnostic accuracy and aid preoperative identification of high-risk patients, enabling restriction of lymphadenectomy for patients with a low risk of aggressive disease.
Endometrial carcinoma is the most common gynecologic malignancy in the Western world, and the incidence is increasing (1). Preoperative risk profile, based on assessment of histologic subtype and grade in endometrial biopsies, combined with imaging methods to detect deep myometrial invasion, cervical stromal invasion, and lymph node metastases is applied to tailor primary surgery. Further assessment of histologic type and grade in hysterectomy specimens and results from surgical International Federation of Gynecology and Obstetrics (FIGO) staging are further used to individualize adjuvant therapy (1–3). These methods are, however, suboptimal, and improved imaging tools to preoperatively exclude lymph node metastases would reduce the need for staging lymphadenectomy, currently frequently performed despite lack of documented survival benefit from the procedure in randomized trials (4). More advanced preoperative risk stratification models could thus safely allow less extensive surgery and reduced postoperative morbidity in low-risk patients (5) and could allow radical hysterectomy or lymphadenectomy to be reserved for the approximately 20% of high-risk endometrial carcinoma patients who follow an aggressive course (4,6).
MR is presently the preferred imaging method for preoperative evaluation of endometrial carcinoma patients (1,7,8), although limitations in the accuracy and reproducibility of MR-based staging parameters have been reported (8–10). 18F-FDG PET/CT combines morphologic and physiologic techniques and is the preferred imaging method for various cancers (11). Although several studies have suggested that 18F-FDG PET/CT can be beneficial preoperatively in endometrial cancer patients (12–23), its clinical role in risk stratification of such patients is not well defined. Furthermore, the value of various quantitative tumor parameters and the corresponding optimal cutoffs for the prediction of tumor stage and prognosis are largely unexplored.
The aim of the present study was to prospectively explore the diagnostic value of preoperative 18F-FDG PET/CT for staging of endometrial carcinoma and to investigate the extent to which 18F-FDG PET–specific quantitative tumor parameters reflect clinical and histologic tumor characteristics. To further explore clinical applicability, we assessed interobserver agreement for 18F-FDG PET/CT staging parameters and quantitative tumor parameters and the prevalence and significance of incidental findings on prospective and consecutive 18F-FDG PET/CT investigations of a population-based endometrial carcinoma cohort.
MATERIALS AND METHODS
Subjects and Study Setting
From October 2011 to November 2013, preoperative whole-body 18F-FDG PET/CT was performed on 129 prospectively included, consecutive patients with endometrial carcinoma. The diagnoses were established through preoperative endometrial biopsy and verified in hysterectomy specimens, and stage was assessed according to the FIGO 2009 criteria for surgical staging. The images were acquired prospectively, and the results were reported to the responsible clinician together with the results of all relevant preoperative imaging. The patients gave written informed consent for the collection of data and specimens for biomarker studies under institutional review board–approved protocols. Image interpretation for staging parameters, reproducibility assessments, and quantifications were conducted retrospectively. All patients were diagnosed and treated at Haukeland University Hospital, a European Society for Gynecologic Oncology–accredited training center for gynecologic oncology, serving a population of about 1 million.
Imaging Protocol
PET/CT was performed on a Biograph 40 True Point scanner (Siemens). Both PET and low-dose CT scanning covered the skull to the proximal thigh. The protocol included 6 h of fasting before image acquisition, and all patients were asked to void before undergoing scanning. 18F-FDG (322–414 MBq) was given intravenously 60–120 min before the CT scan, and the patients rested in a semidark, temperate room between injection and scanning. Low-dose CT (120 kV, 50 mAs) for attenuation correction of the PET data was performed before the static emissions, which were obtained at a rate of 3 min per bed position; immediately thereafter, intravenous contrast agent (Iomerol, 350 mg iodine/mL; Bracco Imaging Scandinavia, AB) and negative oral contrast agent (water) were administered for the subsequent diagnostic CT scan (120 mV, 240 mAs), covering a region from the meatus of the ear to the proximal thigh. The total scanning time was about 25 min per patient. Images were reconstructed and stored in axial, coronal, and sagittal slices 3.0–5.0 mm thick in the department’s PACS (Impax 6; Agfa Healthcare BV).
Data Analyses
A standard imaging report was generated by the responsible nuclear physician and radiologist and reported to the clinical team as part of the routine clinical diagnostic work-up. This imaging report was read and approved by a specialist in nuclear medicine and a radiologist subspecialist in pelvic imaging as part of the standard interpretation setup at our institution.
After being used for routine diagnosis, all images were anonymized, processed, and reviewed retrospectively and independently by 2 physicians experienced in both nuclear medicine and radiology, on a Segami Oasis workstation (version 1.9.4.2; Segami Corp.). Both interpreters had about 4 y of experience with PET/CT before the study. They were unaware of the clinical data and the results of surgical staging, and they reported imaging findings on a standardized form. This form included information on tumor avidity and uptake intensity, as well as metabolic tumor volume (MTV). Information on the presence of increased 18F-FDG uptake in the cervix (interpreted as cervical stroma invasion), in lymph nodes (interpreted as lymph node metastases), and at distant sites (interpreted as likely metastases) was also recorded. The depth of myometrial invasion based on 18F-FDG uptake was not recorded, because the low resolution of the PET signal was perceived to preclude such assessment.
The PET images were fused with both the diagnostic and the low-dose CT images on the Oasis workstation. All measurements were performed using the low-dose fusion images, whereas diagnostic fusion was used for staging. For the measurements of MTV and mean standardized uptake value (SUVmean), voxels with an SUV of more than 2.5 were included in the volume of interest (Fig. 1). Total lesion glycolysis (TLG) in the tumor was also estimated using the following equation: TLG = SUVmean × MTV (24). For the statistical analyses of continuous variables, the mean of the 2 observers’ measurements was applied.
18F-FDG PET/CT images depicting manually drawn MTV in 3 planes: axial (A), coronal (B), and sagittal (C) in 63-y-old woman with FIGO stage 1A endometrioid grade 1 endometrial cancer. In this patient, MTV was 22.4 mL, SUVmax 13.5, and SUVmean 5.5.
To achieve a common understanding of the criteria for assessing tumor avidity, uptake intensity, and MTV, the 2 observers independently recorded 5 selected training cases on the standardized form and then discussed any disagreements or differences in interpretation. These 5 cases were excluded from the study data.
Surgical Staging and Clinical Outcome
In total, 125 (97%) of the 129 patients were surgically staged according to the FIGO 2009 criteria; the responsible physicians of the remaining 4 patients considered them to have inoperable disease, and their diagnoses were based on the uterine biopsies. Depth of myometrial invasion and presence of cervical stromal invasion were assessed macroscopically and confirmed microscopically according to standard procedures. Routine histopathology reports were generated without knowledge of preoperative PET/CT results. Number, size, and localization of lymph node metastases were documented in the histopathology report.
To determine how the clinical team had dealt with follow-up of incidental findings, the medical records were examined retrospectively about 1 y after the PET/CT scans. All additional workups due to the reported incidental PET findings were recorded, together with the results of any repeated examinations.
Statistical Analyses
PET/CT results suggesting cervical stromal invasion or lymph node metastases were compared with the final histopathologic report as the reference standard to calculate sensitivity, specificity, accuracy, positive and negative predictive values, and number of false-positive or -negative findings for each observer and for the imaging report. The relationship between quantitative tumor parameters on PET/CT (SUVmax, SUVmean, MTV, and TLG) and the clinical and histologic tumor characteristics were analyzed by the Mann–Whitney U test, the Jonckheera–Terpsta trend test, and multivariate logistic regression analysis. The intraclass correlation coefficient was used to assess the consistency and reproducibility of the quantitative PET/CT parameters, and the minimal detectable change (1.96 × SEM × ) for these parameters was also calculated.
) for these parameters was also calculated.
Receiver-operating-characteristic (ROC) analyses were performed to evaluate the diagnostic value of the different tumor quantifications in identifying deep myometrial invasion and the presence of lymph node metastases. From these analyses, the optimal cutoffs (rounded to cL) for MTV were estimated by aiming for the values that best separated groups by the Youden index. Statistical analyses were performed using SPSS, version 22.0 (IBM), and Stata, version 12.1 (StataCorp). All reported P values were 2-sided and considered significant when less than 0.05.
RESULTS
Patient Characteristics
Mean age for the 129 patients was 66 y (median, 67 y; range, 26–88 y), and 93% (120/129) of the patients were postmenopausal. Surgical FIGO 2009 staging criteria identified stage IA in 57% (73/129, tumor invading < 50% of the myometrium), stage IB in 17% (22/129; tumor invading ≥ 50% of the myometrium), and unclassified stage I in 1% (1/129). Stage II was detected in 13% (17/129; cervical stromal invasion), stage III in 9% (12/129; local or regional spread), and stage IV in 3% (4/129). Among the 98 patients with the endometrioid subtype, data for grade were available in 92 cases: 57% (52/92) grade 1, 30% (28/92) grade 2, and 13% (12/92) grade 3. Clear cell histology was reported in 5% (6/129), serous histology in 12% (15/129), carcinosarcoma in 5% (6/129), and undifferentiated histology in 3% (4/129). Among the 4 patients with stage IV disease, one had bone metastases; one had abdominal carcinomatosis, including omental metastases; and two had locally advanced tumors with growth into the bladder and rectum, one of whom also had ovarian metastases. All metastases were confirmed by gynecologic examination with ultrasound, MR imaging (bone metastases), biopsy, or perioperative inspection.
Simple hysterectomy with bilateral salpingo-oophorectomy was performed on 91% (118/129) of patients, 7 patients were treated with radical hysterectomy and bilateral salpingo-oophorectomy, and 4 patients underwent palliative procedures (tumor reductive surgery [n = 1] or uterine biopsy [n = 3]). Pelvic lymph nodes were sampled in 75% (97/129), among whom 19% (25/129) also had paraaortic lymph nodes removed as part of the surgical staging procedure. Adjuvant therapy was given to 37% (48/129); chemotherapy to 33% (42/129), pelvic radiation to 3% (4/129), and antihormonal treatment to 2% (2/129).
Diagnostic Performance of 18F-FDG PET/CT in Preoperative Staging
For detecting cervical stromal involvement and lymph node metastases, the observers (including the routine clinical report) had a sensitivity of 25%–33% and 77%–85%, respectively, a specificity of 74%–87% and 91%–96%, respectively, a positive predictive value of 49%–65% and 62%–76%, respectively, and a negative predictive value of 85%–86% and 97%–98%, respectively (Table 1). The 4 cases with confirmed distant metastases were correctly identified and described by observer 1, by observer 2, and in the routine report in 2, 3, and 3 cases, respectively. In a different patient previously treated for breast cancer, widespread malignant disease (metastases in the lungs, mediastinum, bones, and liver) was noted by both observers and in the clinical report, all correctly perceiving this as likely breast cancer metastases in addition to the newly diagnosed localized primary endometrial cancer. No false-positive distant metastases were identified on the basis of PET/CT imaging, yielding a positive predictive value of 100% for both interpreters.
Diagnostic Performance of 18F-FDG PET/CT Compared with Surgical Staging
18F-FDG PET Quantification Measures Associated with Surgicopathologic Findings
The mean SUVmax, SUVmean, MTV, and TLG of the uterine tumors were 14.2, 5.8, 30 mL, and 215 g, respectively, and the corresponding median values were 14.1, 5.7, 19 mL, and 119 g, respectively. All values were significantly higher in tumors microscopically invading at least 50% compared with less than 50% of the uterine wall and in tumors that had lymph node metastases compared with those that did not (Table 2). The same parameters were also significantly higher in high-grade endometrioid tumors, and MTV and TLG were significantly higher in aneuploid tumors. Apart from a significantly higher MTV in tumors with cervical stromal involvement, there was no significant difference in the quantitative parameters related to presence of cervical stroma invasion, histologic subtype, or age (Table 2).
Quantitative Tumor Parameters Assessed by 18F-FDG PET/CT in Relation to Clinical and Histologic Characteristics
SUVmax, SUVmean, MTV, and TLG significantly predicted deep myometrial invasion and lymph node metastases both with and without adjustment for high risk based on preoperative endometrial biopsy (all P ≤ 0.008 and all P ≤ 0.023; Table 3). In contrast, SUVmax, SUVmean, MTV, and TLG did not predict the presence of cervical stromal involvement (Table 3).
ORs for 18F-FDG PET/CT Prediction of Deep Myometrial Invasion, Cervical Stroma Invasion, and Lymph Node Metastases
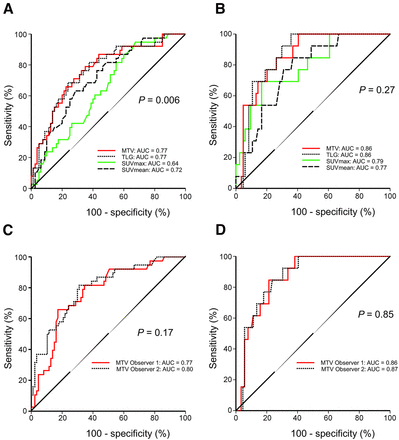
ROC curves showed that MTV had the highest areas under the curve: 0.77 and 0.86 in predicting deep myometrial invasion and lymph node metastases, respectively (Figs. 2A and 2B). On the basis of these ROC curves, an MTV cutoff of 20 mL yielded an odds ratio (OR) of 7.8 (confidence interval [CI], 3.2–19.1; P < 0.001) for deep myometrial invasion, whereas an MTV cutoff of 30 mL yielded an OR of 16.5 (CI, 3.4–80.3; P = 0.001) for lymph node metastases. When adjusting for preoperative biopsy results suggesting high risk (nonendometrioid subtype or endometrioid grade 3), an MTV cutoff of 20 mL yielded an OR of 7.3 (CI, 2.9–18.3; P < 0.001) for deep invasion and an MTV cutoff of 30 mL yielded an OR of 10.9 (CI, 2.1–55.3; P < 0.005) for lymph node metastases.
Receiver-operating-characteristic curves for various tumor quantifications for prediction of myometrial invasion (A) and lymph node metastases (B) and ROC curves for 2 observers for MTV to predict myometrial invasion (C) and MTV to predict the presence of lymph node metastases (D) in patients with endometrial carcinoma. P values refer to test of equal areas under the curve across tumor quantifications. AUC = area under the curve.
Interobserver Agreement
The interobserver agreement for MTV and TLG was moderate, with intraclass correlation coefficients of 0.56 and 0.57, respectively (Table 4). For SUVmax and SUVmean, the intraclass correlation coefficient was very good, yielding values of 0.98 and 0.87, respectively (Table 4).
Interobserver Variability for Tumor SUV and Metabolic Volume Measurements
Incidental Findings
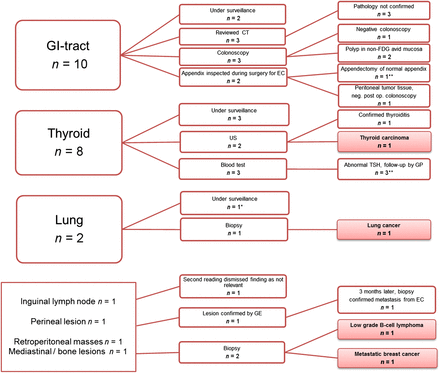
On the basis of the clinical report, significant incidental findings (defined as 18F-FDG uptake with a possible therapeutic consequence) suggesting additional workup were reported for 18% (23/129) of the patients. Further examinations were performed for 17 patients, leading to an additional cancer diagnosis in 4 patients (lung cancer, thyroid cancer, low-grade B-cell lymphoma, and breast cancer) and to the finding of colonic polyps in 2 patients (Fig. 3). For the remaining 11 patients, either no pathologic finding was confirmed (6 patients) or a nonmalignant disease was found and treated (thyroiditis or abnormal thyroid function parameters [4 patients]), the peritoneal 18F-FDG–avid lesion was found to be part of widespread metastatic disease from endometrial cancer confirmed peroperatively (1 patient), or the 18F-FDG–avid perineal lesion was confirmed to be a metastasis from endometrial cancer 3 mo later (1 different patient) (Fig. 3). Six patients are still under surveillance for their incidental findings.
Flowchart of significant incidental findings (defined as 18F-FDG uptake with possible therapeutic consequence) on PET/CT in 129 endometrial carcinoma patients. *One lesion did not have increased uptake. **One patient had 2 separate incidental findings. EC = endometrial cancer; GE = gynecologic examination; GI = gastrointestinal; GP = general practitioner; TSH = thyroid-stimulating hormone; US = ultrasound.
DISCUSSION
Most endometrial carcinoma patients, who typically are elderly, have an excellent prognosis. They are subjected to extensive overtreatment, surgically with lymphadenectomy as a staging procedure, and with adjuvant chemo- and radiotherapy, although the survival benefit from such treatments is undocumented. A follow-up randomized phase 3 trial for women with clinical early-stage high-risk endometrial cancer has been proposed in the STATEC (Selective Targeting of Adjuvant Therapy for Endometrial Cancer) trial (25). The plan is for patients to be randomized to lymphadenectomy versus no lymphadenectomy, followed by adjuvant therapy for node-positive lymphadenectomy patients versus adjuvant therapy for nonlymphadenectomy patients. A sentinel node substudy for patients undergoing lymphadenectomy will compare sentinel lymph node mapping with full lymphadenectomy. It may be a challenge to include preoperative PET/CT in such a study, although further studies also including state-of-the-art advanced imaging methods would be highly valuable in this setting. Including preoperative imaging in future clinical trials could potentially clarify whether advanced imaging tools may contribute to further tailoring of surgical and systemic therapies, which is critical to reduce overtreatment and related side effects.
In the present study, we found that the application of 18F-FDG PET/CT to detect lymph node metastases in endometrial carcinomas yielded sensitivity of 77%–85%, specificity of 91%–96%, and accuracy of 89%–93%. The sensitivity is within the higher range compared with most previous studies (60%–83%) (12–15,22), whereas the specificity is within the lower range compared with previous studies (93%–100%) (12–15,22). Accuracy seems quite similar to that reported recently (90%–95%) (12,13,22). Our ability to compare staging using 18F-FDG PET/CT with that using sentinel lymph node procedures is currently limited, as the true performance in endometrial carcinomas is not well established because the studies have been small and the techniques applied have often varied (26). A recent update from the Memorial Sloan Kettering Cancer Center after 10 y of experience with sentinel lymph node mapping in uterine cancer concluded that prospective studies are needed to validate the use of sentinel lymph node mapping in early-stage endometrial cancer (27). Interestingly, the negative predictive value in the present study was very high (97%–98%), confirming the 95% reported by Criverallo et al. (13) and the 96% reported by Antonsen et al. (22). Thus, the combined high specificity and high negative predictive value confirm that 18F-FDG PET/CT is presently the most promising imaging method to exclude lymph node metastases and thus help avoid potentially harmful lymphadenectomy for staging.
The present study also suggested that measurement of MTV may represent a new tool to assess two well-established surrogate markers for poor outcome: deep myometrial invasion and metastatic lymph nodes; we therefore proposed potential cutoffs to help identify patients at higher risk of having these markers. For deep myometrial invasion, an MTV cutoff of 20 mL yielded sensitivity and specificity of 79% and 68%, respectively, whereas for lymph node metastases a cutoff of 30 mL yielded sensitivity and specificity of 85% and 76%, respectively. Interestingly, this new approach to risk assessment seems to outperform the clinically established method based on histologic subtype and grade in preoperative biopsies, which in this cohort yielded sensitivity and specificity of 47% and 72%, respectively, for deep myometrial invasion and 85% and 66%, respectively, for lymph node metastases. In line with this finding, adjusted for the preoperative biopsy risk assessment, MTV independently predicted deep myometrial invasion and lymph node metastases (Table 3), suggesting that MTV may improve the preoperative identification of high-risk patients and the ability to tailor surgical and systemic therapies accordingly. Our results are in line with a recent study of 56 endometrial carcinoma patients (23) emphasizing MTV and TLG as significant predictors of several clinicopathologic characteristics and superior to SUVmax in differentiating high-risk from low-risk patients. Also two studies of 76 endometrial carcinoma patients each (13,28) suggested that MTV is a promising marker for lymph node metastases and poor outcome. To our knowledge, no previous studies have proposed cutoffs for MTV based on ROC curves predicting deep myometrial invasion and presence of lymph node metastases. As opposed to our proposed cutoff, a recent smaller study of 56 patients proposed a cutoff of 9.4 mL for MTV for differentiation between high-risk and low-risk tumors (based on surgicopathologic assessment). This differing result is possibly explained by a larger proportion of patients with an advanced FIGO stage in the cohort and differing definitions of high- and low-risk groups (23).
All 4 measures for 18F-FDG PET quantification—SUVmax, SUVmean, MTV, and TLG—in the present study were independent predictors of deep myometrial invasion and lymph node metastases after adjustment for high risk based on histologic subtype and grade in preoperative uterine biopsies. The prognostic value of SUVmax assessment has previously been reported for endometrial carcinomas (19,21,23) (n = 101, 268, and 56, respectively), but these studies did not adjust for the routinely applied methods of preoperative risk assessment. A recent review, however, concluded that SUVmax has limited value in risk stratification, although it may aid in the prediction of patient outcome (16). SUVmean, which has been studied less, was found to be associated with FIGO stage, histologic grade, lymphovascular space invasion, and maximum tumor size (similar to SUVmax) in a study of 18F-FDG PET/CT in 60 women with endometrial cancer (17). Our findings that MTV and TLG may be helpful in detecting deep myometrial and cervical stromal invasion are also in line with 3 smaller studies (13,18,23) (n = 76, 84, and 56, respectively).
Interobserver agreement is a critical factor if any biomarker is to become applicable in clinical routine. In the present study, interobserver agreement was moderate for MTV and TLG and very good for SUVmax and SUVmean. This difference was probably due to the subjective steps involved in MTV measurement, where the size of the volume of interest is determined manually in 3 planes. SUV measurements are more robust, as SUVmax depends only on including in the volume of interest the single voxel with the highest value. The interobserver reproducibility of PET-assessed parameters has not previously been examined for endometrial cancer but has been examined for other cancer types, with intraclass correlation coefficients of 0.60–1.00 and 0.85–0.97 being reported for SUVmax and SUVmean, respectively (29–31), which appears to be in line with our findings. Although not directly comparable to our study because of its assessment of whole-body tumor burden, a study of small-cell lung cancer found a similar low interobserver variability, with concordance correlation coefficients of 0.90 for assessment of whole-body MTV (32). Taken together, our data and current literature on other cancer types support the finding that agreement on PET measurements seems lower for volume-dependent parameters than for SUV measurements alone. However, the very similar MTV ROC curves for the different observers (Figs. 2C and 2D) suggest that despite some interobserver variability, MTV may represent a robust imaging biomarker for prediction of deep myometrial invasion and lymph node metastases. Furthermore, the observed agreement for MTV is similar to that reported for other radiologic quantitative methods in daily use within the same field, suggesting that the method may be feasible in clinical routine (33,34).
Detection of incidental findings and second primary cancers on 18F-FDG PET/CT is interesting and not previously reported for a population-based endometrial carcinoma cohort. Similar findings have been reported for cohorts with other primary cancer types (35,36), and the prevalence in our material is at a comparable level. The additional work-up generated by the findings is an important factor in evaluating the cost-effectiveness of 18F-FDG PET/CT, since follow-up examinations are costly and often yield negative results. However, 4 synchronous cancers, all of which had a potentially worse prognosis than the primary endometrial cancer, were diagnosed and treated with curative intent. In addition, a precancerous colonic polyp was successfully removed. Although comprehensive and systematic cost–benefit analyses are difficult in this mixed, casuistic group, we assume that our patients may have particularly benefited from the discovery of incidental pathology on preoperative 18F-FDG PET/CT, leading to intentionally curative treatment that most likely increased their life expectancy and future ability to work.
This study had some limitations; the ROC analyses were conducted a posteriori, and it may be conceivable to prespecify a cutoff by a learning dataset including a smaller number of patients from the same patient population and achieve a priori cutoffs for utter validation (37). However, our patient cohort is presently considerably extended, and we plan to validate the proposed cutoffs in this larger, consecutive patient group.
CONCLUSION
The present study has shown that 18F-FDG PET/CT represents a valuable imaging tool to detect lymph node metastases in endometrial carcinoma patients, and in particular a tool to precisely define patients with a low likelihood of lymph node metastases, in whom lymphadenectomy and adjuvant treatments have no documented survival benefit. All the quantitative parameters assessed were positively correlated with deep myometrial invasion and lymph node metastases. Our proposed approach applying MTV cutoffs outperforms the endometrial biopsy histologic subtyping and grading currently used for preoperative risk stratification.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported by the Western Norway Regional Health Authority; Research Funds from the Department of Radiology, Haukeland University Hospital; MedViz (www.medviz.uib.no), a medical imaging and visualization R&D cluster in Western Norway founded by Haukeland University Hospital, the University of Bergen, and Christian Michelsen Research; the Norwegian Research Council; the University of Bergen; the Meltzer Foundation; the Norwegian Cancer Society (Harald Andersen’s legacy), MedIm (the Norwegian Research School of Medical Imaging); and the Bergen Research Foundation. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jun. 4, 2015.
- © 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication April 23, 2015.
- Accepted for publication May 20, 2015.