Abstract
Accurate definition of the extent and severity of left-ventricular assist device (LVAD) infection may facilitate therapeutic decision making and targeted surgical intervention. Here, we explore the value of 18F-FDG PET/CT for guidance of patient management. Methods: Fifty-seven LVAD-carrying patients received 85 whole-body 18F-FDG PET/CT scans for the work-up of device infection. Clinical follow-up was obtained for up to 2 y. Results: PET/CT showed various patterns of infectious involvement of the 4 LVAD components: driveline entry point (77% of patients), subcutaneous driveline path (87%), pump pocket (49%), and outflow tract (58%). Driveline smears revealed Staphylococcus or Pseudomonas strains as the underlying pathogen in most cases (48 and 34%, respectively). At receiver-operating-characteristic analysis, an 18F-FDG SUV of more than 2.5 was most accurate to identify smear-positive driveline infection. Infection of 3 or all 4 LVAD components showed a trend toward lower survival than did infection of 2 or fewer components (P = 0.089), whereas involvement of thoracic lymph nodes was significantly associated with an adverse outcome (P = 0.001 for nodal SUV above vs. below median). Finally, patients who underwent early surgical revision within 3 mo after PET/CT (n = 21) required significantly less inpatient hospital care during follow-up than did those receiving delayed surgical revision (n = 11; P < 0.05). Conclusion: Whole-body 18F-FDG PET/CT identifies the extent of LVAD infection and predicts adverse outcome. Initial experience suggests that early image-guided surgical intervention may facilitate a less complicated subsequent course.
Left-ventricular assist device (LVAD) implantation improves survival and quality of life in end-stage heart failure and is increasingly used for destination therapy as an alternative to heart transplantation (1–4). Despite continuous technologic improvement, device infection remains a major complication after LVAD implantation (5,6), because the driveline provides a transcutaneous pathway for entry, growth, and spread of pathogens toward internal components of the system and beyond. Although the driveline entry point at skin level is readily accessible, the diagnostic and therapeutic management of internally spreading LVAD infection is challenging.
PET/CT with the glucose analog 18F-FDG, which is avidly taken up by inflammatory leukocytes and infectious pathogens, is an emerging technique for imaging of cardiovascular implant infection (7). The benefit of PET/CT over conventional leukocyte scintigraphy is its higher sensitivity and faster imaging protocol, whereas morphologically driven techniques such as echocardiography and CT are limited by artifacts from the implant material and MRI is not an option. The feasibility and diagnostic accuracy of PET/CT for detecting LVAD infection has been reported in small patient groups by several centers and was recently summarized in a metaanalysis (8). This is a first step toward broader clinical implementation, which should be amended by demonstration of a link between PET/CT results and adverse outcome and by data showing that outcome can be improved when imaging is used to guide therapy (9).
Here, we report the use of 18F-FDG PET/CT for clinical guidance in patients with advanced LVAD infection in our large LVAD referral center (1,4,10). We speculated that 18F-FDG PET/CT not only would identify subjects at elevated risk but also would guide surgical therapy to improve the subsequent course. To test our hypothesis, we obtained clinical information, imaging results, and follow-up in a comparatively large sample of LVAD recipients having undergone PET/CT.
MATERIALS AND METHODS
Study Group
The study group consisted of 57 LVAD recipients, who were consecutively referred for PET/CT between July 2015 and April 2017 for clinical assessment of the extent and severity of device infection due to persisting local or systemic signs of infection. Demographics and other patient characteristics are listed in Table 1. Nineteen patients received repeated scans after 154 ± 11 d of the prior scan (range, 39–343 d) to reevaluate for suspected reappearance or progression of infection. Between scans, there was a change of antibiosis in all but one of the repeat imaging cases, 8 had antiinfective surgery, and 5 had a complete change of the device. The resulting total of 85 scans was used for correlative analysis of image-derived and clinical parameters at the time of PET/CT, when indicated (scan-based analysis). All other analyses, including outcome analyses, were performed on a patient-by-patient basis (n = 57). All patients gave written informed consent to undergo PET/CT imaging. The ethical committee of Hannover Medical School, after being informed of the retrospective analysis of the scan results and clinical outcome, waived the requirement for a formal review.
Patient Characteristics
PET/CT Imaging and Data Analysis
After 6 h of fasting and confirmation that blood glucose levels were less than 8 mmol/L, 262 ± 64 MBq of 18F-FDG were injected intravenously. After an uptake time of 60 min, whole-body PET scans were acquired using a Biograph mCT 128 (Siemens Healthineers) with continuous table motion, as previously described (11). A nonenhanced low-dose CT scan was acquired for anatomic coregistration and attenuation correction. Noncorrected and attenuation-corrected images were reconstructed using a resolution recovery iterative algorithm. Foci of increased 18F-FDG uptake were visually identified by 2 experienced observers. A focus was graded as positive if it was significantly elevated versus background in the regions of the device. Consensus was obtained in cases of discrepancy. All foci of uptake were confirmed as elevated versus background on non–attenuation-corrected images to avoid artifacts induced by dense implant material (12). Using volume-of-interest technique, peak SUVs were obtained from attenuation-corrected scans to quantify the 18F-FDG uptake of each focus. For localization of LVAD-specific infection, the LVAD was subdivided into 4 components (consistent with International Society for Heart and Lung Transplantation recommendations (13)): driveline entry point, subcutaneous driveline pathway, pump pocket, and outflow tract (Fig. 1A). Additionally, SUVs were obtained for regions remote from the LVAD. These included blood pool (aorta) and liver to measure background; hematopoietic organs (spleen, bone marrow), which may be activated in systemic infection; and thoracic lymph nodes with elevated 18F-FDG uptake. Finally, scans were systematically analyzed for other LVAD-related or nonrelated inflammatory foci.
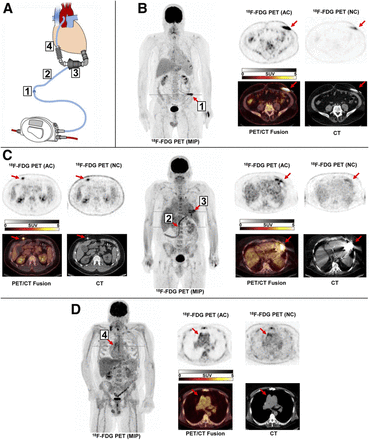
Detection of LVAD infection by 18F-FDG PET/CT. (A) Schematic display of LVAD system, subdivided into 4 components: driveline entry point (1), subcutaneous driveline path (2), pump pocket (3) and outflow tract (4). (B) Patient with infection restricted to driveline entry point at left abdominal wall. (C) Patient with infection of subcutaneous driveline path (2, leftmost panel) and pump pocket (3, rightmost panel). (D) Patient with infection of outflow tract. AC = attenuation-corrected image; MIP = maximum-intensity projection; NC = noncorrected image.
Clinical Data, Driveline Smears, and Follow-up
All LVAD recipients at Hannover Medical School undergo tight clinical follow-up, with outpatient visits at least every 3 mo. Wound smears of the driveline exit side were available at the time of PET/CT, performed by highly experienced nurses according to a standardized protocol. On the basis of this protocol, 99% of all driveline exit sites of newly implanted LVAD patients showed a sterile result, confirming that contamination by normal skin culture is successfully avoided. Regular clinical visits enabled gathering of clinical follow-up data from hospital records, which were obtained for all patients for 2 y after PET/CT or until the occurrence of an event or another PET/CT scan (in cases of scan-based analysis only). Recorded events included death, heart transplantation, LVAD replacement, and any other surgical antiinfective intervention. Additionally, the length of required hospital stays for inpatient care after PET/CT was recorded, and systemic inflammatory markers and smear results from driveline entry points were taken from patient records at the time of PET/CT.
Statistical Methods
Data were analyzed using MedCalc software. Results are reported as average ± SD for continuous variables or as median and range for nonnormally distributed variables. Groups of normally distributed variables were compared using t testing. Nonparametric Mann–Whitney U testing was used if a normal distribution was not confirmed. Receiver-operating-characteristic analysis was used to identify the relationship between SUV at the driveline entry site and the results of microbial smears as a reference. And finally, Kaplan–Meier analysis and log-rank testing were used to identify predictors of outcome during follow-up. A P value of less than 0.05 was considered statistically significant.
RESULTS
18F-FDG PET/CT Identifies the Extent and Severity of LVAD Infection
All scans showed visually detectable foci of 18F-FDG uptake suggestive of LVAD-specific infection. Multiple LVAD components were involved in most scans: 2 in 29 scans (34%), 3 in 28 (33%), and all 4 in 21 (25%). This result confirms the advanced stage of infection in the studied patient group. Most often, elevated 18F-FDG uptake was found along the driveline (entry point, n = 65, 77% of scans; subcutaneous driveline pathway, n = 74, 87% of scans), whereas intrathoracic components were also frequently involved (pump pocket, n = 42, 49% of scans; outflow tract, n = 49, 58% of scans). SUVs for foci at the 4 components (Table 2) showed a decline along the probable pathway of infection, with the highest values being at the driveline entry and the lowest at the outflow tract. Figures 1B–1D show PET/CT images representative of infection for each of the 4 LVAD components.
18F-FDG Uptake at Infected LVAD Components
Driveline entry point smears were available for 83 of 85 studies. Pathogens were identified in 62 scans (75%) and revealed Staphylococcus or Pseudomonas strains in most (respectively: n = 30, 48% of positive cases, and n = 21, 34% of positive cases). Receiver-operating-characteristic analysis revealed an area under the curve of 0.71 (P = 0.004) for detecting a smear-positive driveline entry point infection by elevated 18F-FDG uptake. An SUVpeak of more than 2.5 was identified to be most accurate (Youden index, 0.41), yielding a sensitivity of 87% and specificity of 59%.
On analysis of remote regions, elevated focal 18F-FDG uptake in draining intrathoracic lymph nodes was a frequent LVAD infection–related finding (n = 56, 66% of cases). A representative PET/CT scan showing thoracic lymph node involvement is shown in Figure 2. Of note, the extent and severity of 18F-FDG uptake at LVAD components were not correlated with 18F-FDG uptake in hematopoietic organs or systemic inflammatory markers at the time of PET/CT (C-reactive protein, P = 0.38; leukocyte count, P = 0.34). Sites of 18F-FDG uptake not related to LVAD infection included the sternum, various joints, ribs, and muscles. No septic emboli were identified in the study group.
18F-FDG PET/CT identifies thoracic lymph node involvement in LVAD infection. Shown is patient with infection of subcutaneous driveline path (blue arrows, left, top), pump pocket, and outflow tract (blue arrows, left, bottom). Additionally, mediastinal lymph nodes in upper thorax show elevated 18F-FDG uptake (red arrow, right). AC = attenuation-corrected image; MIP = maximum-intensity projection; NC = noncorrected image.
PET/CT Results Predict Adverse Outcome
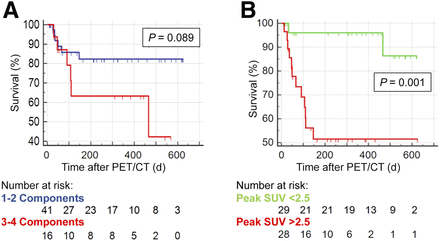
Fourteen of 57 patients (25%) died during follow-up. Death certificates identified sepsis as the underlying cause in 7 patients and multiple-organ failure in 5 patients. Two patients died during or immediately after heart transplantation. The time between the last PET/CT scan and death ranged from 13 to 466 d, with a median of 55 d. Ten patients underwent heart transplantation at 72–334 d after PET/CT (median, 158 d) and were censored at the time of transplantation for survival analysis. Among various image-derived parameters, Kaplan–Meier analysis identified a trend toward adverse outcome if infection involved 3 or all 4 LVAD components (P = 0.089 vs. 2 or fewer components; Fig. 3A). Also, involvement of thoracic lymph nodes was significantly associated with adverse outcome (P = 0.001 for nodal SUV above vs. below median; Fig. 3B). Other markers, including the SUV of any individual LVAD component, the average SUV of all components, uptake in spleen or bone marrow, or detection of non-LVAD infection sites, were not significantly associated with adverse outcome.
Prognostic value of 18F-FDG PET/CT in LVAD infection. (A) Kaplan–Meier curves for patients with extensive infection (3–4 LVAD components) vs. less extensive infection (1–2 components). (B) Kaplan–Meier curves for patients with thoracic lymph node uptake (peak SUV) above median (2.5) vs. below median.
Early Surgical Revision After PET/CT Is Associated with Reduced Inpatient Care
Surgical antiinfective procedures were performed on 32 patients within the first year after PET/CT. These included replacement of LVAD components in 24 patients (at 3–108 d after PET/CT; median, 15 d) and targeted vacuum wound drainage in the other patients. Among those 32, a group of 21 patients received early surgery within the first 3 mo after PET/CT (group 1; surgery assumed to be driven by scan results), whereas a group of 11 patients underwent surgical procedures later than 3 mo after PET/CT, after interval adaptation of medical therapy (group 2; surgery assumed to be independent of scan results). The remaining 25 patients, who did not receive antiinfective surgery, were disregarded for subsequent analysis because they reflect a heterogeneous mixture of patients with less complicated cases (for which continued or adapted systemic antibiotics were considered to be sufficient; n = 14), more severely affected patients who died without surgery (n = 5), or patients who received cardiac transplantation (n = 6).
Survival analysis showed no significant difference between group 1 (5 deaths; 24%) and group 2 (4 deaths; 36%) during follow-up. But the recorded overall length of inpatient care time during the first year after PET/CT differed significantly, showing significantly fewer inpatient days for group 1 (99 ± 87, vs. 158 ± 130 d for group 2, P = 0.016; Fig. 4).
Early surgical revision after 18F-FDG PET/CT is associated with less requirement for inpatient care. (A) Frequency plot for time between PET/CT and subsequent antiinfective surgical procedure. (B) Comparison of inpatient care days in year after PET/CT in subgroups with early and late surgery.
DISCUSSION
Our results confirm that 18F-FDG PET/CT enables detection of the location, extent, and severity of LVAD-specific infection. 18F-FDG also identifies LVAD infection–related involvement of thoracic lymph nodes. Importantly, PET/CT results can be used to identify the subjects at highest risk of an adverse outcome. If targeted surgical revision of infected components is performed early after PET/CT, and thus assumedly guided by imaging results, then the length of subsequent in-hospital stays is significantly reduced. To our knowledge, this report includes the largest single-center sample of 18F-FDG PET/CT scans in subjects with LVAD infection to date. It should be used as a foundation for subsequent prospective trials investigating the effect of PET/CT-guided antiinfective surgical therapy in the management of LVAD recipients with advanced infection.
The instantaneous risk for LVAD infection peaks initially in the postsurgical phase less than 30 d after implantation (5). At subsequent times, it remains constantly elevated at a lower level (5), contributing to a steady increase of cumulative risk with increasing duration of LVAD support. Our study does not cover the early postsurgical phase. Instead, it focuses on the value of 18F-FDG PET/CT in later-onset infections, which are nevertheless known to have a significant effect on survival (5,14,15). Patients included in this analysis were in an advanced state of infection, as confirmed by the high prevalence and extent of positive scans (100% with at least 1 infected LVAD component, 92% with at least 2, and >50% with 3 or 4 components), by the high prevalence of LVAD-related lymph node involvement, by the persistence of infection despite systemic antibiotic therapy before PET/CT, and by the occurrence of all but 1 death within the first 150 d after PET/CT (Fig. 3). This state likely explains our observation that involvement of thoracic lymph nodes as the infection-related drainage site from internal LVAD components had the strongest prognostic value, whereas involvement of internal components themselves showed a trend toward a significant prognostic value but did not have the same discriminatory prognostic power as nodal involvement or as recently reported by another group in a different and smaller sample, for which the focus was on early detection (16).
The state of advanced LVAD infection also represents a situation in which effective therapy is difficult and may most strongly benefit from guidance by imaging. It is generally assumed that LVAD infection spreads from the driveline entry point toward the inner components of the system. A revision or change of the driveline is less complicated and risky than a complete exchange of the LVAD system. However, driveline revision can be a meaningful therapy only if it is done before the infection has reached the aggregate. Here, the comprehensive PET/CT information is useful to guide surgical revision to the infected LVAD components. In our patients, for example, an infection at the aggregate was found in more than 50% of cases, suggesting that a pure driveline-directed therapy would be incomplete for most. Importantly, our analysis also showed that when targeted antiinfective surgery was pursued early after PET/CT, there was a decreased need for inpatient care in the subsequent year when compared with individuals who received surgery at a later time (when the results of PET/CT were not actual anymore). This finding suggests that the greatest value of 18F-FDG PET/CT may lie in guidance of surgical therapy to the appropriate infected LVAD components.
PET/CT had a relevant impact on clinical management in our patient group because it not only guided targeted antiinfective surgery in a subset of patients but also led to prioritization for transplantation in some cases, or to continued or adapted antibiotic therapy, presumably in those patients whose infection was considered less severe. Of note, the exact duration of antibiotic therapy was not completely documented in our retrospective analysis, and repeat imaging was obtained only if recurrence or progression of infection was suspected. Therefore, valid conclusions about the effect of antibiotics on the detection rate of PET/CT cannot be derived from our study.
Some further limitations of our work need to be recognized: First, this was a retrospective single-center analysis, with all the inherent limitations of such. Although the sample size was larger than that of previous original work reporting on PET/CT in LVAD infection, the results still represent the local experience of our referral center. Bias may have been introduced by the clinical use of PET/CT in a more advanced state of infection and by the resulting lack of patients with early or no infection for comparison. The normal distribution of 18F-FDG around the LVAD and its time course after implantation remain poorly defined and cannot be derived from our study group. Also, the length of hospital stay had to be used as a surrogate marker of outcome (17) after antiinfective surgical revision, because differences in survival were not detectable, probably because of the limited power of the still small number of subjects. In addition, the formation of groups based on time between PET/CT and surgery is based on the assumption that an earlier scan will have a more direct effect on surgery, but this assumption cannot be proven because of the retrospective design. Likewise, factors other than time after PET/CT may have confounded the differences in subsequent hospital stay. Therefore, the results of the present study should be seen as a hypothesis generator. This hypothesis—that PET/CT-guided, targeted antiinfective therapy may be beneficial in LVAD infection—clearly needs to be confirmed in a subsequent prospective trial.
Second, this work did not focus on establishing the diagnostic accuracy of 18F-FDG PET/CT in LVAD infection. Most prior studies have focused on the accuracy of the test, suggesting high sensitivity but lower specificity (8,18–20), and superiority when compared with white blood cell scintigraphy (21). Yet, determination of diagnostic accuracy is highly dependent on the validity and robustness of the reference standard (22), which is not free of challenges in LVAD infection, where the proof of pathogens in culture is complicated by sampling error and often longstanding antibiotic therapy (23), and where indirect clinical parameters are proposed as surrogate markers for the presence of infectious pathogens according to International Society for Heart and Lung Transplantation consensus (13). We included an analysis of the driveline entry point as an area that is easily accessible for smears, and we used positive proof of pathogens from smears as the gold standard. Thereby, we were able to determine PET/CT to have high sensitivity and lower but acceptable specificity, both of which were well within the range of prior studies (8). The specificity of 18F-FDG for infection is generally limited, because the tracer does not detect bacteria directly and is taken up by all metabolically active leukocytes. Hence, sterile inflammation cannot be distinguished from infection. The early postsurgical phase, when sterile inflammation is frequently observed because of reparative mechanisms, was excluded from our study. But despite the focus on late-onset LVAD infection, the subjects in our study were treated with antibiotics and may have had local antiseptic wound care, which may have reduced bacteria beyond detectability by smears whereas inflammatory cells taking up 18F-FDG may still have been present. These factors may have further contributed to compromised specificity. Also, we did not include the driveline pathway, pump pocket. or outflow tract in this analysis because structured data on pathogen detection were not available for the internal locations. Nevertheless, although our subanalysis of accuracy may be limited, the major focus of this work was to generate initial data on outcome prediction and modification by imaging and image-guided therapy, and the prognostic value and reduction of inpatient care are observations that stand as they are.
CONCLUSION
The present analysis of a comparatively large patient sample from a single LVAD referral center confirms that 18F-FDG PET/CT identifies the extent of advanced LVAD infection and predicts adverse outcome. Initial experience also supports the notion that early image-guided surgical intervention may facilitate a less complicated subsequent course. The present work provides a foundation for the design of subsequent prospective studies to confirm this hypothesis.
DISCLOSURE
This work was partially funded by the German Research Foundation (Clinical Research Group KFO311: “Advanced Cardiac and Pulmonary Failure”). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can 18F-FDG PET/CT guide surgical therapy in LVAD infection?
PERTINENT FINDINGS: 18F-FDG PET/CT yields accurate information about the presence, severity, and extent of LVAD infection, which predicts adverse outcome. If LVAD recipients undergo surgical treatment early after (and guided by) PET/CT, they require less inpatient care in the subsequent year.
IMPLICATIONS FOR PATIENT CARE: 18F-FDG PET/CT–guided surgical therapy in LVAD infection may improve outcome and reduce complicated patient courses.
Footnotes
Published online Dec. 5, 2019.
- © 2020 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 2, 2019.
- Accepted for publication November 11, 2019.