Abstract
We preoperatively determined the accuracy of 18F-FDG PET/CT for differentiating fixed muscle hypertrophy and fibrotic stenoses from acute transmural inflammatory stenoses in patients with Crohn's disease (CD) scheduled to undergo surgical resection for obstructive symptoms. Methods: Seventeen patients with known CD prospectively underwent 18F-FDG PET/CT before already-planned surgery for obstructive symptoms. Image interpretation was by consensus of 2 readers with knowledge of patient participation in the study but not of other clinical history. Lesions were qualitatively graded on a 5-point scale for the presence of increased 18F-FDG uptake consistent with active inflammation. Maximum lean standardized uptake value (SULmax) was determined for lesions scored 1 or more. Imaging results were compared with the pathologic grading of inflammation and predominant histopathologic subtype for each patient's surgical specimen, whether mainly inflammation, fibrosis, or muscle hypertrophy. Results: Thirteen of the 17 patients underwent surgery (median, 28 d after PET/CT; range, 2–148 d), and 12 of these 13 had histopathologic correlation. Despite the predominant histopathologic subtype (inflammation, 5; fibrosis, 4; and muscle hypertrophy, 3), acute and chronic inflammation, fibrosis (median, 50%; range, 40%−90%), and muscle hypertrophy (median, 20-fold thickening; range, 9- to 40-fold thickening) were found in all patients. SULmax was significantly higher in severe than in mild-to-moderate chronic inflammation (8.2 ± 2.8 vs. 4.7 ± 2.5, P = 0.04). No patient with predominantly fibrosis or muscle hypertrophy (n = 7) had an SULmax greater than 8. Visually, 10 of 12 patients on PET/CT were considered to have active inflammation of the bowel. Conclusion: Patients with CD who undergo surgery for obstructive symptoms have histopathologically mixed findings of inflammation, fibrosis, and muscle hypertrophy. Qualitative PET interpretations were quite sensitive, but additional semiquantitative analyses using SULmax helped identify patients with active inflammation. This information may be beneficial for referring gastroenterologists considering medical therapy versus surgery for patients with CD who present with obstructive symptoms.
Crohn's disease (CD) is a chronic, relapsing inflammatory disorder of uncertain etiology that mostly involves the small bowel or colon. Histologically, early CD is characterized by neutrophilic infiltration of the mucosa, aphthous ulcers, and noncaseating granulomas in one third of patients. Later, aphthous ulcers coalesce and lymphoid aggregates predominate in a characteristic pattern of chronic, transmural inflammation. Muscularis propria hypertrophy and fibrosis occur and contribute to the development of strictures (1). Resection specimens provide complete information on the transmural process.
On the basis of clinical–pathologic behavior, 3 subtypes of CD have been described: stricturing, penetrating, and inflammatory (or nonstricturing/nonpenetrating) (2,3). For patients with the stricturing subtype, inflammation and accompanying cytokines lead to a pattern of muscle hypertrophy and collagen production from myofibroblasts over 8–10 y. This pattern of muscle hypertrophy and collagen production results in luminal narrowing and obstruction. There may be inflammation, ulceration, or fistulization proximal to or within the stricture.
In contrast, a subset of patients with ileal CD presents with active inflammation, diarrhea, and obstruction early after diagnosis, usually in the first 2–4 y (4,5). This subgroup has disease characterized mostly by inflammation, which progresses rapidly and may cause obstructive symptoms (4,5).
Because the narrowing of the bowel lumen caused by acute and chronic inflammation is potentially reversible by medical therapy, differentiating the predominant cause of obstruction in patients with CD has important therapeutic implications. However, focal obstruction is usually due to muscle hypertrophy and constriction with circumferential fibrinous collagen deposition. There is often proximal dilation of the bowel. This is an ideal indication for surgical resection, often done laparoscopically.
Noninvasive anatomic and functional imaging modalities, including CT enteroclysis (6–9), CT enterography, ultrasound, magnetic resonance enterography, and radiolabeled white blood cell and granulocyte scintigraphy (10–19), have shown some ability to measure the extent and severity of active inflammation in CD. Neither these techniques nor endoscopy allows complete assessment of transmural inflammation or extraluminal complications of CD. Important treatment decisions, including surgery or aggressive medical therapy, by the clinician are frequently based on clinical circumstances without imaging confirmation.
Both neutrophils (primary leukocytes of acute inflammation) and macrophages (major cells of chronic inflammation) accumulate high levels of 18F-FDG (20–22). Initial animal and human data suggest that the noninvasive imaging technique using 18F-FDG and PET may help for identifying gastrointestinal inflammation (15,23–28). Combined PET/CT scanners offer more precise lesion characterization and localization (26,27).
Our primary objective was to preoperatively determine the accuracy of 18F-FDG PET/CT for differentiating fixed muscle hypertrophy and fibrotic stenoses from acute transmural inflammatory stenoses in patients with CD who were already scheduled to undergo surgery for obstructive symptoms. The responsible clinician was not aware of the results of the PET/CT research study in terms of degree of inflammation. Secondarily, we compared qualitative and quantitative assessments.
MATERIALS AND METHODS
Study Design and Eligibility Criteria
Our institutional review board approved this prospective study. Informed written consent was obtained from all patients prior to study procedures. Patients older than 18 y with previously documented CD involving the small bowel and Vienna classification L1 (terminal ileum) or L3 (ileocolon) (29), for whom surgery was anticipated within 4 wk for obstructing symptoms, were recruited. None had intestinal fistulas demonstrated preoperatively. Concurrent medications included antibiotics, aminosalicylates, oral prednisone, budesonide, azathioprine or 6-mercaptopurine, and methotrexate.
Exclusion criteria were pregnancy or breast-feeding, creatinine levels higher than 1.5 mg/dL, active infection or serious acute illness, known HIV, known malignancy, diabetes, liver function studies elevated to 3 times normal, hemoglobin levels lower than 8 g/dL, allergies to shellfish or intravenous contrast, estimated survival less than 1 y, and CD activity index (CDAI) greater than 450, which is associated with severe disease (30). Patients who had a stoma created within 6 mo, who had any abdominal surgery within 3 mo of entry into the study, or who required emergency surgery were also excluded. The results of the study were not provided to the referring clinician and did not influence further therapeutic decisions.
Baseline laboratory data, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), were obtained. Baseline CDAI was determined, and each patient received a rating from 1 to 10 for the prediction of the relative degree of inflammation (1) through fibrosis (10) as part of the physician's global assessment (PGA) by the patients' gastroenterologists with expertise in inflammatory bowel disease (IBD) care.
18F-FDG PET/CT Scans
All patients underwent 18F-FDG PET/CT before anticipated surgery. Patients fasted for a minimum of 4 h and had mean blood glucose levels of 88 ± 14 mg/dL before the intravenous injection of a weight-based amount of 18F-FDG (8.14 MBq/kg [mean, 592 ± 148 MBq]). Oral water (n = 14) or 1.3% Readicat-diluted barium contrast material (n = 2) (E-Z-EM Inc.) was administered for the CT portion of the study. One patient could not tolerate any oral contrast material.
After an uptake phase of 18F-FDG of approximately 60 min, a combined PET/CT scan (Discovery LS; GE Healthcare) was obtained from the mid thorax through the pelvis. A non–contrast-enhanced CT scan was obtained first, with a 4-slice multidetector helical scanner and the following parameters: 140 kVp, weight-based amperage (range, 40–160 mA), 0.8 s per CT rotation, pitch of 1.5, and table speed of 22.5 mm/s. A CT transmission map was generated for image fusion. Emission data were acquired for 5 min at each bed position. PET images were reconstructed using the ordered-subset expectation maximization algorithm (2 iterations, 28 subsets), an 8-mm gaussian filter with a 128 × 128 matrix, and CT attenuation correction. After the PET/CT scan, 8 patients also received 120 mL of Omnipaque 350 (GE Healthcare) and underwent venous phase (65- to 70-s delay) intravenous contrast–enhanced CT. Images were obtained from the mid thorax to the pelvis without moving the patient from the imaging table.
Image Analysis
All 18F-FDG PET/CT and intravenous contrast–enhanced CT scans were reviewed in consensus by 2 readers with expertise in PET/CT on a Xeleris workstation (GE Healthcare). Patients' participation in the study of CD with obstruction was known, but the readers were unaware of other clinical information at the time of image interpretation. The PET/non–contrast-enhanced CT scan (PET/CT) was reviewed first, followed by the PET/intravenous contrast–enhanced CT scan (PET/CTintravenous) (n = 8).
Images were reviewed for lesions, defined as foci of 18F-FDG uptake in the abdomen or pelvis potentially representing active inflammation of the bowel. Lesions were scored on a 5-point scale for diagnostic certainty (0, definitely normal; 1, probably normal; 2, equivocal; 3, probably abnormal; and 4, definitely abnormal). A 3-point scale for lesion localization was used (0, unknown; 1, probable; and 2, definite). CT scans with intravenous contrast were considered to provide additional information to PET/CT images if they improved perceived diagnostic certainty for the presence or absence of active inflammation or localization of a lesion.
The secondary objectives of this study were to determine whether semiquantitative analyses of 18F-FDG uptake could be used as a measure of inflammation. Single-pixel maximum standardized uptake value corrected for lean body mass (SULmax) was determined for lesions on PET/CT potentially representing active inflammation of the bowel. SULmax was not determined for lesions that were considered to ultimately represent physiologic findings on PET/CT. For those patients with more than 1 lesion on 18F-FDG PET/CT, a representative lesion with the highest SULmax and best correlating with findings at surgery was chosen for further analysis.
Total lesion (tumor) glycolysis was previously described and reflects both tumor metabolism and size, and changes in total lesion glycolysis are potentially valuable for monitoring response of a tumor to therapy (31). Accordingly, we calculated total inflammatory volume (TIV) for each patient, hypothesizing that TIV might be more representative of a patient's overall inflammatory burden than single-pixel SULmax.
On the basis of a technique used for total lesion glycolysis in colorectal cancer (32), TIV was calculated by the product of PET-derived inflammatory volume (PETinf-vol) and the average SUL in the PETinf-vol. The lesion of interest was first defined with 3-dimensional software tools available on the Advantage Workstation (GE Healthcare). Then, PETinf-vol was derived using a thresholding tool and the patient's average SUL in a 3-cm region of interest in the liver as the lower limit of 18F-FDG uptake.
For 8 patients with PET/CTintravenous, average Hounsfield units (HU) were determined in a 1-cm region of interest around a lesion's region of SULmax on the non–contrast-enhanced and contrast-enhanced CT scans. Percentage change in HU was determined by:
Surgery
All patients were expected to require surgery for obstructive symptoms and agreed to surgery on enrollment into the study. The decision to proceed with surgery was made by the gastroenterologist and surgeon on the basis of the patient's disease longevity and severity of obstructive symptoms, presence of clinical disease activity, and anatomic imaging findings and endoscopy. The entire bowel was examined intraoperatively and areas of stricture or obstruction assessed and resected.
The gastroenterologist and surgeon were unaware of the results of the PET/CT scans, unless unexpected extraabdominal findings were seen that might have emergently affected patient care. One patient had a 1.4-cm lung nodule with intense 18F-FDG uptake, and pathology from wedge resection performed before bowel resection revealed granulomatous inflammation.
Histopathology
Slides were reviewed by 1 of 3 experienced gastrointestinal pathologists who were unaware of the results of the PET/CT scans, using a previously validated histopathologic scoring system developed as part of this protocol (results will be reported separately).
Surgical specimens were received fresh from the operating room and immediately processed according to previously published methods (33). Blocks, at least 1 per 5-cm length, were taken from all diseased areas, from resection margins, and from areas that appeared normal. All focal areas of narrowing were examined. A single slide from the thickest and most involved area of each individual block was analyzed.
Paraffin tissue sections were evaluated for the degree of inflammation with hematoxylin and eosin stains. The degrees of acute neutrophilic infiltration and chronic lymphoplasmacytic infiltration were scored as absent (0), mild (1), moderate (2), or severe (3), based on the density and extent of inflammatory infiltrate and bowel-wall thickening seen. The global inflammation score was calculated as the sum of the acute and chronic inflammation scores.
Paraffin tissue sections were evaluated for collagen with trichrome staining for fibrosis scoring. The percentage of fibrosis involving the muscularis propria was determined in the most fibrotic affected area. Measurements (in 10% increments) were made on the basis of the density, intensity, and extent of involvement.
The tissue sections were evaluated for muscle hypertrophy with hematoxylin and eosin stains. Morphometric measurements were obtained of the diameter of thickest hypertrophied muscularis propria and compared with the diameter of the normal-appearing muscularis propria in the same patient (i.e., hypertrophied muscularis propria diameter/normal muscularis propria diameter).
Finally, the predominant histopathologic subtype for each patient was determined by the pathologist as mainly inflammation, fibrosis, or muscle hypertrophy. The final predominant histopathologic subtype considered findings from the gross specimen and all 3 scoring evaluations (inflammation, fibrosis, and muscle hypertrophy).
Statistics
Kruskal–Wallis tests were used for the comparison of means. Correlations between SULmax, TIV, percentage change in HU, and clinical data were performed using Pearson correlation coefficients and z tests (Systat and Microsoft Excel software). P values less than 0.05 were considered statistically significant.
Receiver-operating-characteristic curve (ROC) analysis was performed using JROCFIT software (http://www.rad.jhmi.edu/jeng/javarad/roc/JROCFITi.html) to determine potentially optimal values of SULmax and TIV for accurately diagnosing active inflammation of the bowel.
RESULTS
Seventeen patients (9 women, 8 men; median age, 39 y; range, 26–63 y) with CD were enrolled. Demographics and baseline clinical and laboratory data are presented in Table 1. Six patients underwent surgery within 14 d of PET/CT (median, 28 d; range, 2–148 d). Four patients recruited for the study underwent PET/CT but recovered from obstructive symptoms and did not require surgery.
Demographics and Clinical and Laboratory Results
PET/CT Findings
Thirty-three foci of increased 18F-FDG uptake (lesions) in the abdomen that potentially represented active inflammation in the bowel were visualized on PET/CT. All 17 patients had at least 1 lesion (median, 2; range, 1–3). For the presence of active inflammation, diagnostic certainty scores were definitely abnormal (4) for 18 lesions, probably abnormal (3) for 5, equivocal (2) for 3, probably normal (1) for 3, and definitely normal (0) for 4.
Considering the single lesion with the highest 18F-FDG uptake per patient, the average SULmax (±SD) of potential active CD inflammatory lesions was 6.9 ± 2.9. The average TIV for all patients was 172 ± 136 SUL·mL.
For 8 patients with both PET/CT and PET/CTintravenous, the addition of intravenous contrast did not result in significant alterations of the PET/CT interpretation. The same 14 PET findings were seen on both PET/CT and PET/CTintravenous. Three diagnostic certainty scores increased from 3 to 4, and there were no changes in any lesion localization certainty scores. Probably or definitely abnormal lesions on PET/CT localized to thickened bowel wall with mucosal and wall enhancement after intravenous contrast. The average percentage increase in HU of corresponding (to PET findings) bowel wall after intravenous contrast was 162% ± 81% (range, 55%−318%).
Pathologic Findings
Twelve of 13 patients who underwent surgery had pathologic correlation (Table 2). The predominant histopathologic finding was inflammation in 5, fibrosis in 4, and muscle hypertrophy in 3. One patient (of 13) who underwent surgery had a bypass of the Crohn's stricture via a jejunojejunostomy rather than resection because of the finding of cryptogenic cirrhosis with ascites and a history of poor healing. Tissue for histopathology was not obtained in this patient, but at surgery a chronic stricture in the jejunum was found, resulting in dilation of proximal small bowel.
Histopathology and PET/CT Results
Some degree of acute inflammation was found in all patients (n = 4, mild; n = 2, moderate; and n = 6, severe). All patients also demonstrated chronic inflammation (n = 1, mild; n = 3, moderate; and n = 8, severe). All patients demonstrated muscle hypertrophy of the muscularis propria (median, 20-fold thickening; range, 9- to 40-fold thickening). The median percentage fibrosis of the muscularis propria was 50% (range, 40%−90%).
Correlation of PET/CT with Histologic Inflammation
For PET/CT, 4 of 5 patients with predominantly active inflammation on histology were believed to have active inflammation (diagnostic certainty scores, 3 or 4). One patient had an equivocal diagnostic certainty score (2) for active inflammation. Moderate or severe acute and chronic inflammation was found on histopathology in all 5 patients with predominantly active inflammation.
Patients with severe chronic inflammation had significantly higher SULmax than those with mild or moderate chronic inflammation (8.1 ± 2.8 vs. 4.7 ± 2.5, P = 0.04) but not higher TIV (192 ± 146 vs. 161 ± 182 SUL·mL, P = 0.73). Examples are shown in Figures 1 and 2. On the basis of acute (Table 3) or global inflammation scores (data not shown), no significant differences in SULmax or TIV were observed.
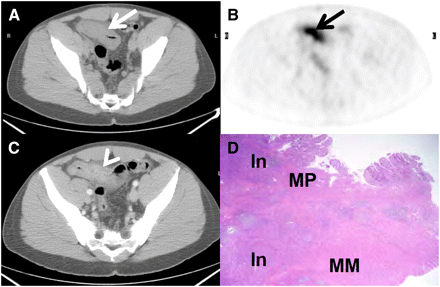
A 33-y-old man (patient 12) with CD for 3 y presented with cramps after eating. Small bowel series and CT scan showed marked thickening of distal ileum with luminal narrowing and proximal dilation of bowel. (A and B) PET/CT revealed intense 18F-FDG uptake fusing to thickened ileum (arrows). Diagnostic certainty score for active inflammation was 4 (definitely inflammation). SULmax was 13, and TIV was 258.2 SUL·mL. (C) Intravenous contrast–enhanced CT scan shows mural stratification (arrowhead). (D) In resected terminal ileum, there was acute and chronic transmural inflammation (In) and muscularis propria (MP) and muscularis mucosa (MM) hypertrophy and fibrosis.
A 31-y-old woman (patient 3) with CD for 10 y and 3 prior small bowel obstructions presented with right lower quadrant pain and was diagnosed with recurrent partial small bowel obstruction. (A–C) PET/CT demonstrated small focus of 18F-FDG uptake fusing to normal-appearing distal small bowel on non–contrast-enhanced CT scan (arrows). Diagnostic certainty score was 1 (probably not inflammation), SULmax was 2.5, and TIV was 5.1 SUL·mL. At ileocecectomy, terminal ileum and cecum were moderately indurated, and ileocecal area had narrow stenosis. (D) In resected terminal ileum, there was marked muscularis propria (MP) and muscularis mucosa (MM) hypertrophy and fibrosis but minimal acute or chronic inflammation.
Semiquantitative Analyses of 18F-FDG Uptake
Accuracy of 18F-FDG PET/CT for Detecting Inflammation
ROC curve analyses of PET semiquantitative analyses revealed cutoff values of 8.0 for SULmax and 223.6 for TIV as optimal for distinguishing inflammation and fibrosis or hypertrophy in the bowel (Figs. 3 and 4). For SULmax greater than 8.0, the sensitivity for detecting active inflammation of the bowel was 60%, specificity was 100%, positive predictive value was 100%, negative predictive value was 78%, and accuracy was 83%. For TIV greater than 223.6, the sensitivity was 60%, specificity was 71%, positive predictive value was 71%, negative predictive value was 60% and accuracy was 67%.
ROC curves of SULmax (A) and TIV (B). Area under curve for SULmax is 0.78 and 0.67 for TIV. On the basis of ROC analysis, optimal cutoff values yielding best diagnostic accuracy for presence of active inflammation in bowel were 8 for SULmax and 223.6 SUL·mL for TIV.
Correlation of SULmax (A) and TIV (B) for individual patients vs. predominant histopathologic subtype. Using cutoff value of 8.0 for SULmax (line), sensitivity for detection of active inflammation of bowel was 60%, specificity was 100%, positive predictive value was 100%, and negative predictive value was 78%. Using 223.6 (line) as cutoff value for TIV, sensitivity was 60%, specificity was 71%, positive predictive value was 71%, and negative predictive value was 60%.
No patient with an SULmax less than or equal to 8 was classified as having acute inflammation as their predominant histopathologic subtype. In 2 cases, acute inflammation was associated with an SULmax less than 8. In 1 patient (patient 11), the time between the PET scan and surgery was delayed to 42 d for work-up of hydronephrosis found incidentally on imaging. The cause of the low 18F-FDG uptake in the second patient (patient 7) with predominantly inflammation remains unclear.
Sensitivity, specificity, and accuracy were not calculated on the basis of visual diagnostic certainty scores for the presence of active inflammation by PET and PET/CT because nearly all (8/12 on PET alone and 10/12 on PET/CT) patients had scores of 3 or 4 (probably or definitely active inflammation).
For 14 lesions in the 8 patients with PET/CTintravenous, no significant correlations were found between percentage change in HU in the bowel wall after contrast and SULmax or TIV.
Lack of Correlation of PET/CT with Predominant Histologic Pattern
Seven patients had predominantly fibrosis or muscle hypertrophy on histology. Regardless, 6 of these 7 patients had moderate or severe chronic inflammation and 3 had moderate or severe acute inflammation. Diagnostic certainty scores of 3 or 4 suggesting active inflammation were assigned to 6 patients by PET/CT. One patient had a predominant histopathologic subtype of muscle hypertrophy and had a score of 1, suggesting the absence of active inflammation.
SULmax was not significantly different for patients with fibrosis or hypertrophy and inflammation (6.0 ± 2.2 and 8.3 ± 3.9, P = 0.22; Table 3). There were no significant differences in TIV between patients with fibrosis or hypertrophy and inflammation, though the mean for the inflammation group appeared higher (136 ± 135 and 245 ± 164 SUL·mL, P = 0.37).
The number of days between the PET/CT scan and surgery tended to be longer in patients with fibrosis or hypertrophy than in those with inflammation (52 ± 52 d vs. 21 ± 24 d). The number of days between the PET/CT scan and surgery did not correlate with SULmax (r = 0.04, P = 0.90) or TIV (r = 0.07, P = 0.83).
Lesion Localization on PET/CT
Lesion localization on 18F-FDG PET/CT was compared with the location of the resected lesion at surgery for 18 lesions with a diagnostic certainty score greater than or equal to 1. Two rectosigmoid lesions and a perianal fistula were not examined or resected at surgery. Seven lesions were correctly localized by 18F-FDG PET/CT to the terminal ileum with or without cecal involvement. Seven lesions that involved the terminal ileum and cecum or ileocecal valve were localized to the distal small bowel only on 18F-FDG PET/CT. One lesion that was localized to the ileocecal valve on 18F-FDG PET/CT involved only the small bowel on resection.
Lack of Correlation of Laboratory Data with Histology and PET/CT
No significant correlations were found between clinical parameters (PGA, CDAI, ESR, or CRP) and SULmax or TIV. On the basis of predominant histopathologic subtype (fibrosis or hypertrophy vs. inflammation), there were no significant differences in PGA (7.4 ± 1.3 vs. 6.6 ± 0.89, P = 0.18), CDAI (166 ± 80 vs. 230 ± 52, P = 0.22), ESR (16 ± 13 mm/h vs. 24 ± 21 mm/h, P = 0.46), or CRP (0.92 ± 1.1 mg/dL vs. 2.1 ± 1.6 mg/dL, P = 0.17). The median CDAI score for the 12 patients with histopathology was 205. Neither SULmax nor TIV was significantly different between those with a CDAI score less than 205 or greater than 205 (6.1 ± 2.6 vs. 7.9 ± 3.5, P = 0.63, and 141 ± 118 vs. 222 ± 181, P = 0.63, respectively).
DISCUSSION
We compared the level of 18F-FDG uptake on PET/CT to the histopathology of resected specimens of bowel from patients with CD and obstructive symptoms, hypothesizing that acute inflammatory lesions would accumulate more 18F-FDG than would predominantly fibrotic strictures or muscle hypertrophy. Our results demonstrated that although 18F-FDG uptake correlates with the presence of inflammation in lesions of CD, fibrotic strictures and muscle hypertrophy also accumulate 18F-FDG. Evaluation of the entire bowel wall via surgical specimens was a unique and crucial aspect of the present study for understanding the pathophysiology of the 18F-FDG uptake in our patient population.
The inability of 18F-FDG PET/CT to discriminate between predominantly active inflammatory lesions, fibrotic strictures, or muscle hypertrophy by visual interpretation alone was probably attributable to the continuum of both acute and chronic inflammatory cells in all patients' specimens. The primary leukocytes in both acute and chronic inflammation (neutrophils and macrophages, respectively) are well known to accumulate high levels of 18F-FDG (20–22). Patients with severe chronic inflammation had significantly higher SULs than those with mild or moderate inflammation, supporting the mechanistic aspects of PET in active inflammation.
Another plausible explanation for our high false-positive rate by visual interpretation is a contribution of 18F-FDG uptake by contracting or hypertrophied smooth muscle in the bowel wall. The foci of increased 18F-FDG uptake considered representative of active inflammation localized mostly to the bowel wall, not intraluminally, on the coregistered CT scan, and substantial muscle hypertrophy was seen in all histopathologic specimens. Previous attempts to elucidate the precise etiology of 18F-FDG uptake by the bowel were limited and yielded mixed results, but several authors reported decreased 18F-FDG uptake in the bowel after the administration of antiperistaltic agents, at least suggesting a smooth muscle component (34,35).
Semiquantitative analyses of 18F-FDG uptake helped distinguish predominantly active inflammation from fibrotic strictures and muscle hypertrophy. An SULmax cutoff of 8 seemed to be most accurate. The higher (but not statistically significant) mean TIV in predominantly active inflammation suggests the possibility that this parameter is also informative, but TIV was considerably variable. This higher mean TIV may reflect a bias toward inflammation in all patients in this study. The relatively small number of patients in whom histology was obtained limits the statistical power of this observation.
Interestingly, patients with predominantly active inflammation, compared with those with predominantly fibrosis or muscle hypertrophy, underwent surgery closer to the time of PET/CT. These patients may have clinically been more symptomatic, requiring surgery sooner. By the time of surgery, some component of active inflammation visualized on 18F-FDG PET/CT could have partially resolved for those with predominantly fibrosis or muscle hypertrophy.
Several studies have suggested that 18F-FDG PET and PET/CT in adult and pediatric patients, compared with standard endoscopy, histology, radiography, and small bowel imaging, are useful for identifying active IBD (15,23–28). Sensitivities for this purpose have been reported to range from 54% to 98%, with specificities from 55% to 81% (15,23–28). In general, these studies were limited because they did not provide a full evaluation of the entire bowel wall, and CD lesions are characteristically transmural.
In a prospective study of 22 patients with CD, Louis et al. (26) reported an overall sensitivity of 72.9% and a specificity of 54.2% for detecting active CD lesions in 95 bowel segments evaluated by 18F-FDG PET/CT and endoscopy performed for medical reasons. 18F-FDG PET was 100% sensitive for the detection of severe lesions (deep ulcers and strictures). We also observed increased 18F-FDG uptake in fibrosis or hypertrophy and found some type of intestinal pathology that correlated with the need for surgery in all of our patients. Differentiation between stricture and acute inflammation was not attempted in the study by Louis et al. (26).
Numerous studies have demonstrated that increased leukocyte accumulation in active IBD correlates well with disease extent and severity in CD, compared with endoscopy, histology, and fecal excretion of 111In-leukocytes (10–19). For inpatients with symptoms of small bowel obstruction (a population somewhat similar to ours, though probably with more severe active disease because 76% of our patients were outpatients) and positive enteroclysis, a negative 111In-leukocyte scan was reported to be suggestive of chronic fibrostenotic stricture, not acute inflammatory disease, due to lack of response to medical therapy (18).
The existence of the noninflamed stricture has recently been challenged. In 48 patients with CD, 31 of 34 bowel strictures were positive for inflammation on histopathology from laparotomy, and 25 (74%) of these were positive on leukocyte scintigraphy (11). In pathologic specimens collected at our institution, CD strictures had a mean inflammatory score of 2.3 (maximum inflammation score, 3), with 83% demonstrating at least moderate inflammation and 47% having severe inflammation (Theodore M. Bayless, unpublished data, 2006). Our data further support these findings.
We did not find any significant correlations between any of the clinical parameters (PGA, CDAI, ESR, CRP) and SULmax or TIV for predicting the presence of fibrosis or hypertrophy versus inflammation. This is in concordance with the findings of some authors (11,15) but not others (23,26). It is possible that the smaller range of CDAI scores in our patient population (60–304) was not large enough to show differences in 18F-FDG uptake based on this parameter. Also, obstructive rather than active disease symptoms led to inclusion in the current study. In a study including 176 patients with CD, CRP level at diagnosis was not found to be associated with location and biologic behavior of disease, but higher CRP levels were associated with increased risk for surgery in the subgroup with disease in the terminal ileum (36).
CT enterography or enteroclysis may have a role in determining whether small bowel narrowing is due to active inflammation (6–9). On CT enteroclysis, active inflammation is suggested by mural and wall enhancement, mural stratification, lymphadenopathy, engorgement of vessels, and wall thickness; fibrostenotic strictures, however, are associated with nonenhancing wall thickening, proximal dilation, and luminal stenosis (6). We did not apply semiquantitative or qualitative criteria for reading the intravenous contrast–enhanced CT scans as done in other studies (6–9).
Limitations of this study were the small study population and the readers' knowledge of the patients' diagnosis of CD and participation in the study. These factors could have biased the readers to be more sensitive to the detection of any abnormal 18F-FDG uptake in the bowel, which is often variable. No patients without CD were included as controls. However, the lack of a control group probably did not alter the results for the specific question being addressed in this study (fibrosis or hypertrophy vs. inflammation in patients with CD and obstructive symptoms) but limited determination of true specificity for detection of CD by 18F-FDG PET/CT. The exact correlation between the location of lesions visualized on PET/CT and resected at surgery was difficult, and standardization of pathology remains a challenge.
Finally, the lack of correlation between the predominant histopathologic subtype and established clinical markers of active inflammation at least raises the question of the validity of “predominant histopathologic subtype” as the gold standard. This study demonstrates that there is most likely a continuum of multiple processes involved in the etiology of luminal narrowing in patients with CD.
CONCLUSION
Patients with CD who undergo surgery for obstructive symptoms have histopathologically mixed findings of inflammation, fibrosis, and muscle hypertrophy. 18F-FDG PET/CT SULmax was significantly higher in severe inflammation. Although qualitative PET interpretations using our scale appeared to be too sensitive for detection of inflammation, semiquantitative analyses using SULmax helped identify patients with predominantly active inflammation. This information may be helpful for referring gastroenterologists considering surgery versus medical therapy for patients with CD who present with obstructive symptoms. Further studies in larger patient populations are needed and could also examine the role of PET/CT in assessing the efficacy of medical management in treating IBD.
Acknowledgments
We thank Judy Buchanan and Donna Rode for their assistance in the preparation of this manuscript. Support for this study was provided by the Broad Medical Research Program, grant BMRP IBD-0122R.
Footnotes
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication April 24, 2009.
- Accepted for publication July 21, 2009.