Abstract
The use of 99mTc-ciprofloxacin as a tracer for infection and inflammation has been examined and discussed in the literature extensively. Its alleged ability to discriminate between sterile inflammation and bacterial versus nonbacterial infections has led to an intense debate. Other labeled fluoroquinolones might offer better characteristics or may add to a better understanding of the working mechanism of 99mTc-ciprofloxacin. The rationale of this work was to determine possible differences in the use of 2 labeled quinolones—that is, 99mTc-ciprofloxacin and 99mTc-enrofloxacin—as tracers for infection and inflammation in animals. Methods: Ciprofloxacin and enrofloxacin were labeled with 99mTc and characterized. The stability of both preparations was evaluated in serum and in the presence of an excess of cysteine. In vitro binding studies were performed to determine the interaction of the labeled quinolones with bacteria and other cells. Rats with sterile and infectious intramuscular lesions were used to study the scintigraphic properties of the 2 compounds. To assess the specificity of binding to living bacteria, infectious intramuscular lesions of heat-killed Staphylococcus aureus and Candida albicans were used as controls. Imaging was performed with a γ-camera at 0, 3, 5, and 22 h after injection. Results: The radiochemical purity of both radiolabeled fluoroquinolones exceeded 95% as determined by instant thin-layer chromatography. Both compounds were moderately stable in serum. Binding assays did not show any saturable binding to S. aureus, heat-killed S. aureus, as well as C. albicans. None of the tracers showed specific binding to bacteria. Scintigraphy showed uptake in the infectious lesion at 1 h after injection, which washed out during the next 4 h. Abscess-to-muscle ratios for both preparations were not significantly different for the various infectious or inflammatory lesions studied and did not exceed an average of 4.25 ± 0.62. Conclusion: The study demonstrated that 99mTc-ciprofloxacin and 99mTc-enrofloxacin do not show preferential binding to living bacteria. In vivo 99mTc-enrofloxacin has similar characteristics as 99mTc-ciprofloxacin except for differences in uptake in a few normal tissues.
Ciprofloxacin labeled with 99mTc has been presented as a radiopharmaceutical that could differentiate between bacterial infection and nonbacterial infection or sterile inflammation (1). Being able to discriminate between bacterial infection and other inflammations with a simple scintigraphic procedure could have a major impact on the clinical management of patients with suspected bacterial infection.
99mTc-Ciprofloxacin has been widely tested in clinical studies and has been the subject of an intense debate (2–6). Besides the alleged ability to distinguish bacterial infection from nonbacterial inflammation, the compound has many other advantageous characteristics, such as availability, low costs, fast blood clearance, and an easy labeling procedure (7).
Ciprofloxacin is a fluoroquinolone antibiotic. The mechanism of action of these fluoroquinolones is not fully understood, but it has been postulated that the interaction of ciprofloxacin with bacterial DNA gyrase (a type II topoisomerase) prevents DNA uncoiling and subsequent DNA synthesis (8–10). The structures of ciprofloxacin and enrofloxacin are very similar (Fig. 1). Both compounds demonstrate a significant antibiotic effect for both gram-negative and gram-positive bacteria and are active in both stationary and growth phases of bacterial replication. Because of their structural similarity, we hypothesized that enrofloxacin can be labeled with 99mTc in a similar manner, provided that both substituted nitrogen atoms do not affect the complexation. 99mTc-Enrofloxacin might have different characteristics than 99mTc-ciprofloxacin and may add to a better understanding of the overall working mechanism of 99mTc-ciprofloxacin.
Molecular structure of ciprofloxacin (R = H) and enrofloxacin (R = CH3).
In the present study, the ability of both 99mTc-ciprofloxacin and 99mTc-enrofloxacin to visualize soft-tissue infections or sterile inflammations in rats was investigated. The stability of the radioligands was characterized, in vitro binding assays were performed, and scintigraphic imaging of inflamed thigh muscle tissue with these compounds in rats was studied.
MATERIALS AND METHODS
Tracers
Ciprofloxacin (Bayer) was labeled with 99mTc essentially as described by Sonmezoglu et al. (4) with minor modifications. Briefly, 99mTc-ciprofloxacin was prepared by mixing 500 μg stannous tartrate (Sigma-Aldrich) (reducing agent) with 400 MBq freshly eluted sodium pertechnetate in a vial. Directly thereafter, 0.5 mL of a 4 mg/mL ciprofloxacin solution was added. The vial was shaken and heated at 60°C for 10 min. Subsequently, the preparation was purified using a SepPak tC2 light cartridge (Waters) and was eluted with 1 mL of ethanol:water (1:1). Enrofloxacin (Bayer) was labeled in exactly the same way.
High-Performance Liquid Chromatography (HPLC) Analysis
The compounds were further characterized by HPLC analysis using an Alltima C18 column (Alltech) connected to an ultraviolet-visible (UV-VIS) spectrometer (254 nm) (SPD-6AV; Shimadzu) and a NaI γ-counter (model 2200; Ludlum), eluted with 50 mmol/L triethylaminophosphate, pH 2.25 buffer (eluent A), and a MeOH (eluent B) gradient. The elution was as follows: 0–3 min, 100% eluent A; 3–6 min, from 100% to 75% A; 6–9 min, from 75% to 66% A; 9–20 min, from 34% to 100% B; 20–27 min, 100% B; and 27–30 min, from 100% B to 100% A.
Instant Thin-Layer Chromatography (ITLC) Procedure
To determine the radiochemical purity of 99mTc-ciprofloxacin, a 1-μL sample of the preparation was spotted on 2 silica gel-impregnated ITLC strips (Gelman Laboratories). To determine the pertechnetate content of the preparations, 1 strip was developed using acetone as the mobile phase. In this system, pertechnetate migrated with the front of the mobile phase (Rf = 1.0). To determine the colloid content of the preparations, the second ITLC strip was developed using ethanol:water:ammonium hydroxide (2:5:1) as the mobile phase. In this system, the colloid is found at the origin of the strip (Rf = 0).
Stability Test
The stability of the 99mTc-quinolone preparations was determined in human serum at 37°C as previously described by Steffens et al. (11). Serum samples were analyzed by ITLC at 0, 2, 18, and 24 h of incubation as described. In addition, the stability of the preparations was tested in a challenge assay. Equimolar solutions of the tracers were incubated with increasing concentrations of cysteine (10−4, 10−3, 10−2, 0.1, 1, 10, and 100 mmol/L). During 4 h of incubation at 37°C, the stability of the 99mTc-quinolone preparations was determined at several time points by ITLC.
Microorganisms
Staphylococcus aureus 6538 (S. aureus) and Candida albicans 10231 (C. albicans) were obtained from the American Type Culture Collection. Overnight cultures of S. aureus bacteria were prepared on tryptone soja agar (Oxoid) plates at 32°C. C. albicans were cultured on Sabouraud’s dextrose agar (Oxoid) during 3–5 d at 22°C. Aliquots of suspensions of harvested microorganisms containing 2 × 108 colony-forming units (cfu) were kept in 1 mL of buffered peptone water (Oxoid) at 4°C for a maximum of 1 wk.
In Vitro Cell Binding Studies
Increasing amounts of both tracers were added to 1 mL of incubation buffer (7.52 g/L K2HPO4, 1.32 g/L NaH2PO4·H2O, 7.2 g/L NaCl, 0.5% bovine serum albumin, pH 7.8) containing 1 × 107 cfu S. aureus, 1 × 107 cfu heat-killed S. aureus, and 1 × 107 cfu C. albicans. Simultaneously, incubations were performed in the presence of an excess of unlabeled antibiotic (45 μg/mL) to determine the nonspecific binding. All binding assays or incubations were performed in at least 3-fold unless indicated otherwise. After a 2-h incubation at room temperature, the cells were centrifuged at 3,500g for 6 min, the supernatant was removed, and the pellet was washed once with 1 mL of the binding buffer. The pellets were counted on a multiwell NaI γ-counter (Cobra; Packard). To verify whether the unlabeled compound in the incubation mixture had not affected the viability of the microorganisms, a suspension from each series was additionally washed and cultured on their respective testing plates.
Thigh Muscle Infection Model
In 2 groups of 20 Wistar rats (body weight,180–220 g) inflammation was induced in the left thigh muscle on day 1. Of each group, 5 rats were infected with S. aureus (5 × 108 cfu), 5 were infected with heat-killed S. aureus (5 × 108 cfu), 5 rats were injected with turpentine, and 5 rats were infected with C. albicans (1 × 108 cfu). One day later, when the swelling of the inoculated muscle was apparent, the rats were injected intravenously with 7 MBq 99mTc-enrofloxacin or with 7 MBq 99mTc-ciprofloxacin. During the experiments animals obtained food and water ad libitum. All animal studies were approved by the local Experimental Animal Ethical Committee and performed in accordance with their guidelines.
Scintigraphy and Biodistribution
Biodistribution of the radiolabel was determined by γ-camera imaging at 0, 3, 5, and 22 h after injection. Three rats of each group were placed prone on a single-head γ-camera (Orbiter; Siemens Medical Systems) equipped with a parallel-hole, low-energy, all-purpose collimator. Images were obtained with a 15% symmetric window over the 140-keV energy peak of 99mTc. After acquisition of 100,000 or 300,000 counts, the images were stored in a 256 × 256 matrix and processed with an ICON image-processing system. During scintigraphy the animals were anesthetized with halothane.
Twenty-four hours after injection of the radiolabeled compounds, the rats were killed by carbon dioxide suffocation. Blood was obtained by cardiac puncture. Tissue samples (right muscle, infected left muscle, lung, spleen, kidney, liver, and intestine) were dissected and weighed, and their activity was measured in a shielded well-type scintillation γ-counter (Wizard; Pharmacia-LKB). To correct for physical decay and to calculate uptake of the radiolabel in each tissue sample as a fraction of the injected dose, aliquots of the injected dose were counted simultaneously. The results were expressed as percentage injected dose per gram (%ID/g). Abscess-to-blood and abscess-to-muscle ratios were calculated.
Postmortem Microorganism Cultivation
The microorganisms present in the infected muscle tissues were determined. The left thigh muscle from the rats was aseptically removed, 2 mL of phosphate-buffered saline were added, and the tissue was homogenized using a tissue homogenizer (T18 basic; Ultra Turrax) (45 s, 9,000 rpm). Serial 10-fold dilutions were brought into the respective testing plates and incubated, after which the cfu were counted. As a control, tissues of uninfected and sterile inflamed thigh muscle were examined simultaneously.
Statistical Analysis
All mean values are expressed as %ID/g or ratios ± 1 SEM. Data were analyzed statistically using an unpaired t test (GraphPad Instat 3.05 for Windows 95/NT; GraphPad Software, Inc.).
RESULTS
Radiolabeling
Using the described protocol, both compounds were labeled with labeling efficiencies of 72% ± 7% and 81% ± 4% for enrofloxacin and eiprofloxacin, respectively. Specific activity was calculated, and average values were 160 MBq/mg for 99mTc-ciprofloxacin and 120 MBq/mg for 99mTc-enrofloxacin.
Radiochemical Purity
Quality control with ITLC before separation of colloids indicated that the 99mTc-ciprofloxacin preparation contained <5% pertechnetate (TcO4−) and <10% colloids (TcO2). For 99mTc-enrofloxacin, the pertechnetate content was similar, but the amount of colloid in the preparation was much higher (up to 30%). Both preparations were purified using a C18 cartridge, and the radiochemical purity of both radiotracers was >95%.
HPLC
The UV chromatogram and the radiochromatogram of both tracers were similar. A UV chromatogram and a radiochromatogram of 99mTc-enrofloxacin prepared according to the standard protocol is shown in (Fig. 2). The UV chromatograms showed 1 UV peak with a retention time of 17.7 min. This same peak was found on the radiochromatogram. The activity peak at 13.2 min represents free TcO4−.
(A) Radiochromatogram of 99mTc-enrofloxacin. (B) UV chromatogram of enrofloxacin.
Stability Tests
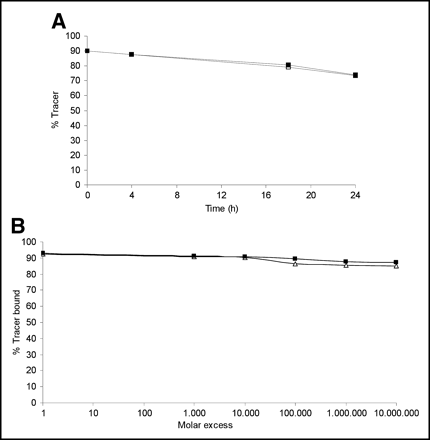
During incubation in human serum, both compounds were not fully stable as determined by ITLC (Fig. 3A). Up to 16% of free radiolabel was found at 24 h of incubation at 37°C. In a challenge exchange assay in buffer in the presence of increasing amounts of cysteine (1–107 mol/L), the compounds lost <5% of radiolabel after 4 h of incubation as determined by ITLC (Fig. 3B).
(A) Stability of 99mTc-enrofloxacin (□) and 99mTc-ciprofloxacin (▪) in human serum at 37°C followed in time. (B) Stability of 99mTc-enrofloxacin (▵) and 99mTc-ciprofloxacin (▪) in challenge exchange assay using increasing molar amounts of cysteine.
In Vitro Binding Studies
Both preparations did not show saturable binding to 1 × 107 cfu living or heat- killed microorganisms because the binding could not be blocked with an excess of unlabeled ciprofloxacin. Nonspecific binding was relatively low (3%–6%). Most important, no significant differences were found for binding to S. aureus, heat-killed S. aureus, and C. albicans with either compound. Binding percentages were equal for microorganisms incubated with a 50-fold excess of unlabeled ciprofloxacin or enrofloxacin (up to 2.4 × 103 Bq/107 cells), indicating that neither compound bound specifically to any of the microorganisms tested (Fig. 4). With the binding assays, no significant differences were found between the 2 tracers. After 2–3 h of incubation, neither the labeled nor the unlabeled antibiotics affected the viability of cell suspensions of S. aureus or C. albicans.
(A) Binding percentage of 99mTc-enrofloxacin to different microorganisms with (▪) or without (□) excess of unlabeled antibiotic. (B) Binding percentage of 99mTc-ciproloxacin to different microorganisms with (▪) or without (□) excess of unlabeled antibiotic.
Scintigraphy and Biodistribution Studies
One day after inoculation of the microorganisms in the calf muscle, 2 groups of 20 rats were injected with 0.1 mL of 7 MBq 99mTc-enrofloxacin and 99mTc-eprofloxacin. Three rats of each group of 5 animals, injected with S. aureus, heat-killed S. aureus, turpentine, or C. albicans, were scanned at 0, 2.5, 5, and 22 h after injection (Fig. 5). Intense renal activity was observed. Uptake in the inflamed muscles was noted very early after injection in all rats (time = 0 h), but at 5 h after injection this activity had cleared from the infected or inflamed calf muscle. The uptake of 99mTc-ciprofloxacin in the liver, spleen, and lungs was lower than the uptake of 99mTc-enrofloxacin in the same organs. Table 1 shows target-to-background ratios obtained from region-of-interest analysis of 99mTc-cirpofloxacin at several time points after injection. At 0, 2.5, and 5 h after injection, a slow decrease of the target-to-background ratios was observed in time. At 22 h after injection, the target-to-background ratios are less reliable due to physical decay and excretion of the radiolabel. No visual difference between infected and contralateral thigh was observed at this time.
Scintigraphic images of 99mTc-ciprofloxacin (A) and 99mTc-enrofloxacin (B) in rats with thigh muscle infections at different time points (infections or inflammation is located in right thigh in images).99mTc-Enrofloxacin shows higher accumulation in liver and spleen.
Target-to-Background Ratios from ROI Analysis of 99mTc-Ciprofloxacin at Various Times After Injection
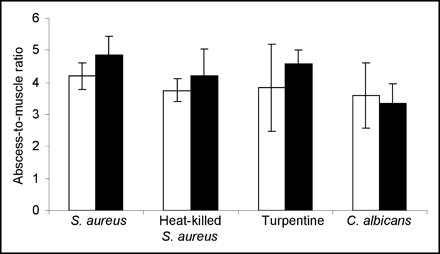
Biodistribution data are shown in Table 2. The tracers both showed high uptake levels in the kidneys. 99mTc-Enrofloxacin showed higher uptake in the spleen, liver, and lungs than did 99mTc-ciprofloxacin. Uptake in the various inflamed muscle tissues tested was similar for both tracers. For 99mTc-enrofloxacin, the mean ratio of infected muscle tissue to healthy muscle tissue was 4.2 ± 0.6. For 99mTc-ciprofloxacin, this ratio was 3.8 ± 0.8 (Fig. 6).
Abscess-to-muscle ratios of 99mTc-enrofloxacin (□) and 99mTc-ciprofloxacin (▪) in thigh muscle–infected rats at 22 h after injection.
Biodistribution Data of 99mTc-Labeled Quinolones in Infected Wistar Rats 22 Hours After Injection
Microorganism Culturing
After injecting 5 × 108 cfu in rats on day 1, postmortem (day 3) counting of viable microorganisms in dissected infected tissue samples was always positive. Values were generally >0.5 × 107 cfu/g tissue for all organisms. In addition, all infectious sites contained pus, indicating active infectious processes. Aseptically removed tissue of uninfected and sterile inflamed thigh muscle resulted in negligible viable counts of the various organisms.
DISCUSSION
In this study, ciprofloxacin and enrofloxacin were labeled with 99mTc with high radiochemical yields and with high specific activities (120–160 MBq/mg). During labeling of both fluoroquinolones, some 99mTc-colloids were formed, which could be effectively removed by elution on a C18 cartridge. Both labeled compounds were moderately stable, with a slow release of radiolabel during incubation in serum. A release of up to 16% of the radiolabel after a 24-h incubation was observed.
The antimicrobial activity of fluoroquinolones is directed toward gram-negative and gram-positive bacteria, not toward yeasts such as C. albicans. The idea of labeling fluoroquinolones is to preserve the capacity to bind bacteria and thereby enable the compound to specifically target those microorganisms. No binding to living or heat-killed bacteria was found. Similarly, no specific interaction was found between 99mTc-fluoroquinolones and C. albicans. Possibly this is a result of vital changes in the conformation of the molecules or modification of the active sites of these molecules due to complexation with technetium. The effect of a 99mTc nucleus on the properties of small molecules such as fluoroquinolones could be considerable. The carboxyl function combined with the keto function is thought to participate in the coordination of the technetium. Yet, these functions as well as the fluor atom are thought to interact with the bacterial topoisomerase (8–10). Most likely, the addition of the 99mTc atom to this part of the ciprofloxacin molecule will affect the interaction of the molecule with the enzyme.
Because these antibiotics can exhibit their antimicrobial activity in both stationary and growth phases of bacterial replication, it could be argued that bacteria were killed during the in vitro binding assays, explaining the minimal interaction with the microorganisms in vitro. However, this hypothesis could be ruled out, by showing that the bacteria were still alive after the in vitro binding assays.
Our studies in rats with intramuscular infection or inflammation indicated that the uptake in the inflamed tissue could not be ascribed to specific binding to living bacteria; thus, in this study we could not establish the basis for the potential of 99mTc- ciprofloxacin to distinguish bacterial from nonbacterial infection. The radiolabeled compound, therefore, could not distinguish bacterial infection from sterile inflammation. No significant differences were found in uptake at in vivo sites of infection or inflammation. Rats with infectious lesions injected with 99mTc-enrofloxacin showed a mean abscess-to-muscle ratio of 4.25 ± 0.62. For 99mTc-ciprofloxacin, mean values were 3.84 ± 0.82. These findings are in accordance with the results obtained from the in vitro binding assay. The rapid uptake seen on images at 0 and 1 h most likely is due to the physiologic changes at the site of infection. Increased local blood supply together with increased vascular permeability may enhance transudation of the radiolabeled compounds at the inflammatory sites, explaining the abscess-to-muscle ratios, which were larger than unity, in each of the experimental groups (12). Kleisner et al. (13) reported on a novel synthesis and conclude that, in experimentally induced infection with S. aureus, the amount of accumulated 99mTc-ciprofloxacin activity was 5 times higher than that in controls at 2 h after injection, which is accordance with our findings for both tracers.
The higher uptake of 99mTc-enrofloxacin in normal tissues such as liver and spleen probably is due to the higher lipophilicity of this compound (14).
Our studies are in line with previous reports describing the selective uptake of radiolabeled ciprofloxacin in infectious and inflammatory lesions (15). Sarda et al. (16) showed that 99mTc-ciproloxacin in clinical studies was unable to discriminate septic arthritis or osteomyelitis from aseptic osteoarticular diseases. Similarly, a lack of specificity was found in a rabbit model of S. aureus prostethic joint infection (17).
CONCLUSION
In this rat model of intramuscular infection and sterile inflammation, neither 99mTc-ciprofloxacin nor 99mTc-enrofloxacin was able to discriminate between infection and sterile inflammation. This finding was supported by data from in vitro binding studies in which no significant differences in binding for the microorganisms were found. Except for variations in uptake of the tracers in liver, lung, and spleen, probably due to the presence of colloids in the preparation, 99mTc-enrofloxacin did not differ from 99mTc-ciprofloxacin in any aspect.
Acknowledgments
The authors thank Gerry Grutters (Central Animal Laboratory, University Medical Center Nijmegen) and Cathelijne Frielink (Department of Nuclear Medicine, University Medical Center Nijmegen) for their excellent technical assistance in the animal experiments.
Footnotes
Received Apr. 7, 2004; revision accepted Jul. 21, 2004.
For correspondence or reprints contact: Rien H. Siaens, MSc, Harelbekestraat 72, 9000 Ghent, Belgium.
E-mail: Rien.Siaens{at}ugent.be