Abstract
The objective of this prospective study was to determine the extent to which the levels of thyroid-stimulating hormone (TSH) influence the uptake of FDG by thyroid carcinoma tumors Methods: Ten patients with follicular (n = 7) or papillary (n = 3) thyroid carcinoma underwent FDG PET during TSH suppression (<0.05 μU/mL) and TSH stimulation (>22 μU/mL) within an average interval of 42 d (range, 28–73 d). The findings were evaluated by visual criteria. In addition, a tumor-to-background ratio (TBR) was determined for 17 lesions that were visualized. Results: In 15 of 17 lesions with positive FDG uptake, TSH stimulation was associated with an increase in the TBR from 3.85 ± 2.53 (mean ± SD) to 5.84 ± 4.84, corresponding to an average increase of 63.1% (P < 0.001). Determination of absolute counting rates indicated that this increase was the result of a decrease in FDG metabolism in the background together with an increase in the tumor tissue. No relationship was found between the presence or absence of iodine storage capacity (5 versus 12/17 lesions) and increase in FDG accumulation. Seven of 10 patients had additional iodine-positive metastases that showed no accumulation of FDG. Conclusion: Most locally recurrent and metastatic follicular and papillary thyroid carcinomas exhibited a significant increase in FDG uptake on TSH stimulation. In 3 of 10 patients, TSH stimulation resulted in either detection of new lesions or classification of the FDG uptake pattern as typical for malignancy. These findings suggest that FDG uptake in recurrent and metastatic thyroid carcinoma depends on the TSH level. Therefore, we recommend that PET examinations be performed in patients with thyroid carcinoma under TSH stimulation and follow-up examinations be performed under identical TSH conditions to prevent erroneous interpretation.
The value of PET using FDG in the diagnosis of recurrent and metastatic disease, particularly for the detection of 131I-negative tumor manifestations, is generally accepted. It has been observed that iodine uptake of a tumor is inversely related to its glucose metabolism. Hence, 131I scintigraphy and FDG PET are complementary diagnostic methods. In several studies, the sensitivity and specificity of FDG PET were equal to or even higher than that of human serum thyroglobulin (hTg) and have shown high predictive values in patients with elevated hTg levels and negative findings on 131I whole-body scintigraphy (1–5). In these series, the various tumor manifestations in an individual patient accumulated either exclusively 131I or exclusively FDG or showed a hybrid pattern with accumulation of both. This uptake behavior has been observed in both papillary and follicular thyroid carcinomas. The heterogeneity of 131I and FDG uptake may correspond to various degrees of differentiation of individual disease manifestations. On the one hand, a correlation between the degree of differentiation of thyroid carcinoma cells and their organ-specific uptake of iodine has been postulated. On the other hand, analogous to other malignancies, the progressive loss of cell differentiation has been associated with an increase in FDG metabolism (6). This assumption is supported by observations showing a relationship between the grading of the primary tumor and the intensity of FDG uptake in thyroid carcinomas (6). An increase in FDG metabolism in more aggressive disease forms has been described for other types of malignancies (7,8).
Whether the level of thyroid-stimulating hormone (TSH) influences the FDG uptake in differentiated thyroid carcinoma cells has yet to be determined. Comparable FDG PET findings in 131I-negative metastases have been reported by Grünwald et al. (4), who studied patients almost exclusively under TSH suppression, and Dietlein et al. (5), who included patients only under TSH stimulation. Sasaki et al. (9) also studied carcinoma patients under different TSH conditions but did not observe any relationship between FDG uptake and TSH levels. However, to our knowledge, FDG accumulation has not been compared intraindividually in patients with thyroid cancer under TSH suppression and TSH stimulation. A sequential examination of a single patient has been reported by Sisson et al. (10) in a case report. Visual criteria and quantitative parameters showed increased FDG metabolism under TSH stimulation in this patient.
Possible dependencies of FDG uptake on TSH levels are of clinical importance and may lead to serious misinterpretations—for example, in the evaluation of response to therapy. Therefore, the objective of this prospective study was to investigate the influence of different TSH levels on the uptake of FDG in locally recurrent and metastatic differentiated thyroid carcinoma.
MATERIALS AND METHODS
Ten patients (6 men, 4 women; mean age, 69.8 y; age range, 62–82 y) were recruited for this prospective study. Using the TNM classification system (11), at the time of primary diagnosis 7 patients had follicular carcinoma and 3 had papillary carcinoma. (T4/T3/T2/T1, n = 5/0/4/1; N2/N1/N0/NX, n = 0/2/5/3). All biopsy specimen showed typical follicular and papillary histologies, respectively. Distant metastases were identified in 9 of 10 patients before inclusion in the study. With the exception of 1 patient, who had undergone only one course of 131I therapy (3.7 GBq), all patients had received multiple administrations of 131I (average applied activity, 32.2 GBq). In all patients, high levels of hTg suggested persistent disease. The average time interval between thyroidectomy and inclusion in this study was 45.9 mo. All patients underwent FDG PET under TSH suppression (<0.05 μU/mL) and, after withdrawal of exogenous thyroxine, under TSH stimulation (>22 μU/mL). Normal reference values for TSH in our laboratory are 0.3–4.0 μU/mL. The average time interval between the two PET examinations was 42 d (range, 29–73 d). At least 90 d had elapsed between prior 131I therapy and the first PET examination. To determine whether iodine uptake capacity was preserved, all patients underwent diagnostic whole-body scintigraphy 48 h after oral application of 370 MBq 131I. With one exception (patient A, Table 1) all patients underwent 131I therapy within 1 wk after the diagnostic scanning and the PET study during hypothyroidism. Whole-body 131I scintigraphy (whole-body scanning) after therapy was performed 72 h after oral administration of 3.7–9.2 GBq 131I. All patients gave informed consent. The study was approved by state and federal authorities as well as by the University institutions.
Data of 10 Patients with Metastatic Thyroid Carcinoma
PET studies were performed using an ECAT-Exact HR+ scanner (CTI/Siemens, Knoxville, TN). The scanner simultaneously acquires 63 contiguous transverse sections of 2.46-mm thickness covering an axial field of view of 15.5 cm with one bed position. Patients fasted for at least 6–8 h before the examination. FDG was injected intravenously at a dose (mean ± SD) of 180 ± 10.5 MBq (range, 165–198 MBq). Intraindividual variation in the applied FDG activity and in the time elapsed until the beginning of measurements (mean, 73 min) was no more than 3% and 10%, respectively. With one exception (141 mg/dL, value measured under TSH stimulation), blood glucose levels were in the range of 71–118 mg/dL (TSH suppression, 82 mg/dL [mean]; TSH stimulation, 83 mg/dL [mean]). To prevent increased FDG uptake in the area of the larynx, patients were instructed not to speak from 10 min before to 30 min after application of the radiopharmaceutical. All PET examinations conducted under TSH suppression were done from the base of the skull to the proximal segment of the femora in three-dimensional mode (five–eight bed positions, 7 min per bed position). Examinations under TSH stimulation were conducted only in the regions of known or suspected tumor manifestations in two or three contiguous bed positions. Transmission scanning and correction for attenuation of the emission data were not done.
Emission data were reconstructed by a filtered backprojection reconstruction algorithm using a Hanning filter at a cutoff frequency of 0.4 Nyquist. The in-plane resolution (full width at half maximun) for reconstructed data was 7 mm in the center of the field of view.
Evaluation of PET studies was primarily done visually on the monitor and on hard-copy films. In addition, for 17 visually detected lesions, a tumor-to-background ratio (TBR) was calculated under both TSH conditions. For TBR evaluations, identical regions of interest (ROIs) were drawn at both examinations on hot spots representing the area of highest accumulation in transversal sections. Also, identical ROIs for each serial study were placed over contralateral areas or, in the case of midline findings (e.g., local recurrent disease), over adjacent areas representing unaffected soft tissue. Calculation of the TBR was performed using mean values of the counting rate within the ROI.
Data are expressed as mean ± SD. Differences in the groups were compared using the Mann-Whitney U test. To compare the intraindividual results, the Wilcoxon rank test was used. P < 0.05 was considered significant.
RESULTS
The TBR was calculated under both TSH conditions in 17 lesions that showed increased FDG uptake. Foci of pathologic FDG uptake were located in the thyroid bed (n = 2), neck (n = 2), bone (n = 2), and lung or pulmonary hilus (n = 11). A change in the size of individual areas of FDG uptake was not observed as a result of TSH stimulation.
In 15 (131I uptake present in 4 and absent in 11 lesions) of 17 lesions, TSH stimulation resulted in an increase in the TBR from 3.85 ± 2.53 to 5.84 ± 4.89 (P < 0.001). On the basis of all data, a mean increase in the TBR of 63.1% was registered.
In 8 lesions with intense FDG uptake during TSH suppression, visual findings did not change recognizably under TSH stimulation. In the other 7 cases, there was a significant visual increase in FDG uptake intensity during TSH stimulation compared with TSH suppression. However, in 4 of these cases, the pattern of FDG uptake under TSH suppression was consistent with malignancy; hence, despite the increase in uptake intensity, there was no change in putative diagnosis. On the other hand, in the 3 remaining lesions (3 patients), TSH stimulation resulted either in the first visualization of a pattern of FDG uptake consistent with malignancy or in a focus of formerly discrete FDG uptake now being recognized as suggestive of malignant disease.
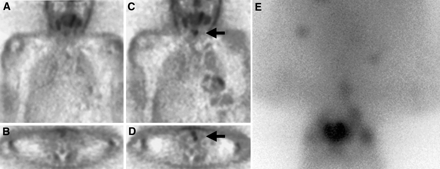
In the first of these patients (patient G), no pathologic pattern of FDG uptake was identified under TSH suppression. On the second PET scan obtained 31 d later during hypothyroidism, with a TSH level of 79 μU/mL, there was an area of FDG uptake in the left thyroid bed consistent with malignancy (increase in TBR, 86%). This corresponded with a soft-tissue mass detected by CT, resulting in a diagnosis of suspected local recurrence. The subsequent 131I whole-body scan revealed several pulmonary manifestations and one mediastinal tumor manifestation, all FDG negative. However, no relevant 131I uptake was observed in the thyroid bed itself (Fig. 1). Local recurrent disease was verified by biopsy 1 mo later.
FDG PET (A–D) and 131I scintigraphy (E) of patient G (Table 1). (A and B) Unsuspicious findings under TSH suppression with thyroxine. (C and D) After 31 d, note findings suggestive of malignancy in left paralaryngeal area (arrow) under conditions of hypothyroidism. (E) Normal visualization of pharyngeal mucosa and salivary glands with identification of several pulmonary and one mediastinal metastasis right of midline (all FDG negative). No evidence of 131I uptake is seen in projection onto PET findings. Local recurrent disease was verified histologically 1 mo later.
In the second patient (patient E), an unusual pattern of FDG uptake was identified in the pelvic region during TSH suppression. It was not interpreted as consistent with malignancy because of its low intensity. The second scan obtained 29 d later during hypothyroidism provided a clear diagnosis with an increase in the TBR of 63%. The subsequent 131I whole-body scan revealed several FDG-negative metastases, but the area of FDG uptake in the pelvis also showed an intense 131I accumulation (Fig. 2).
FDG PET (A–E), CT (F), and 131I scintigraphy (G) of patient E (Table 1). (A–C) Under TSH suppression, note unusual configured uptake pattern in right pelvic area (arrow). Moderate uptake intensity corresponds with that of surrounding intestinal tissues. (D and E) With TSH stimulation, note increase in uptake intensity consistent with malignancy; configuration of lesion is unchanged. (F) Osteodestruction of lateral part of right sacrum as well as osteolysis on left caused by soft-tissue lesion. (G) Intensive 131I uptake in right and left halves of sacrum, in hepatic vault, and in lung. There is no evidence of FDG metabolism in latter tumor manifestations.
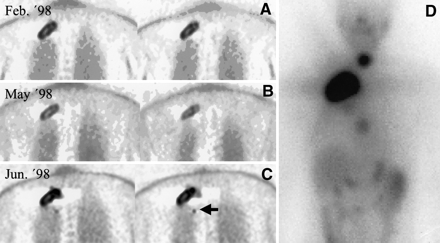
A previous PET examination under hypothyroidism (TSH, 66 μU/mL) was available for the third patient (patient B). This study revealed an intense, centrally photopenic pattern of FDG uptake in the right apical thoracic wall. The patient underwent 131I therapy with 7400 MBq immediately thereafter. Three months later, a PET scan obtained under l-thyroxine substitution (TSH < 0.05 μU/mL) showed a significant decrease in uptake intensity. This was first taken as a sign of positive response to therapy. However, the follow-up PET examination 28 d later (TSH, 60 μU/mL) showed FDG uptake intensity comparable with the initial findings, with an increase in the TBR of 127% compared with the TSH suppressive state. In addition, a small lesion adjacent to the large tumor was seen, which had been visible previously only on 131I scintigraphy. Because the hTg levels in the period between the first and third PET scans had not increased markedly (+8%), the patient was considered to be in a phase of stable to questionably progressive disease. An 131I whole-body scan confirmed a retained 131I uptake capacity of the metastasis in addition to revealing an FDG-negative metastasis in a thoracic vertebra and recurrent disease in the thyroid bed (Fig. 3).
FDG PET (A–C) and 131I scintigraphy (D) of patient B (Table 1). (A–C) Metastatic follicular thyroid carcinoma with FDG uptake on three consecutive PET scans. (A) Scan, obtained in state of hypothyroidism immediately before planned 131I therapy, shows intense FDG metabolism in known metastasis of thoracic wall. (B) Scan was acquired 3 mo later when patient was receiving oral thyroxine. (C) Scan, obtained 4 wk later under TSH stimulation, shows pattern of intense FDG uptake comparable with patient's initial findings. In addition, note first evidence of small satellite focus (arrow). (D) 131I whole-body scan confirmed retained 131I-uptake capacity of metastasis described. In addition, FDG-negative metastasis in thoracic vertebra and recurrent disease in thyroid bed are shown.
In 2 (131I uptake present in 1 lesion and absent in 1 lesion) of 17 lesions, TSH stimulation was associated with moderate decreases in the TBR of 6.6% and 9.3%, respectively. However, visual findings were not changed recognizably by these findings (patient H, Table 1).
No correlation was found between present and absent 131I uptake capacity (5/17 versus 12/17) and increase in FDG metabolism under conditions of TSH stimulation (mean, 73.4% versus 58.8%). The mean hTg values (reference range, >0.5 ng/mL) were 2982 ng/mL (range, 27–16,868 ng/mL) under TSH suppression and 6301 ng/mL (range, 124–21,780 ng/mL) under TSH stimulation. As expected, TSH stimulation resulted in an increase in hTG values, but no significant correlation was found between the percentage increase in hTg and the percentage increase in the TBR (r = 0.25).
In papillary carcinomas, the mean TBRs under TSH suppression and TSH stimulation were 2.98 and 5.16; in follicular carcinomas, the mean values were 4.32 and 6.21, respectively, but this difference was not significant because of the small number of patients. The TBR change in different metastases of the same patient was also of interest. The increase in different lesions of individual patients ranged from 8.0% to 73.4% and 8.1% to 83.8% (patients A and I).
To determine whether the increase in the TBR under TSH stimulation was caused by an absolute increase of FDG uptake in malignant tissue or by hypometabolism of FDG in surrounding tissue, we determined the absolute counting rate in the tumor and the background ROIs under both TSH conditions. In 11 of 17 tumor ROIs measured, the counting rate under TSH stimulation was higher than that under TSH suppression (range, +7.8% to +95.7%), whereas the remaining 6 ROIs (4 patients) showed a lower counting rate (range, −17.5% to −31.5%). The average increase for all tumor ROIs was 19.3% (not significant). A slight increase in the counting rate (range, +0.4% to +14.2%) was observed in the background ROI only in 5 of 17 measurements, whereas in most cases a considerable decrease in the counting rate (range, −15.4% to −61.7%) was detected. This corresponds to a mean decrease of the counting rate in background ROIs of 21.6% (P < 0.01). Note that in all 6 tumor ROIs with a decreasing counting rate, the background counting rate in general was decreasing.
DISCUSSION
The findings of this study confirm observations of Sisson et al. (10) in a single case study suggesting an effect of TSH levels on the FDG metabolism of metastases of differentiated thyroid carcinoma. In our patients, we observed a significant increase in the TBR (63.1%) under TSH stimulation compared with the measurement under TSH suppression with exogenous thyroxine in the same patients. In 3 of 10 patients, patterns of FDG uptake consistent with malignancy were observed only on TSH stimulation. However, there was considerable overlap of measurement results, such that, in the individual case, it was impossible to determine whether a given measurement had been made under conditions of TSH stimulation or TSH suppression. This could also explain why other researchers, who did not obtain sequential PET scans, were unable to show a clear-cut influence of TSH on FDG uptake (3,4).
The observed changes in FDG parameters cannot be explained by statistical variations of repetitive measurements. Repetitive measurements of FDG uptake in different solid tumors have been associated with variations of only 8%–14% (12). In our study there were only minor intraindividual variations in the net applied FDG dose (≤3%) and the time interval between application and beginning of measurements (≤10%). This, combined with stable blood glucose levels, makes any major random errors unlikely in our study. The only more elevated glucose level was measured under TSH stimulation and therefore would have led to a decreased FDG uptake (and therefore to a decrease in TBR) rather than to an increased FDG uptake. It is also unlikely that the increase in the TBR was associated with progression in tumor size. With one exception, metastatic disease had been recognized in all patients for at least 15 mo before examination, and all but one patient had undergone multiple courses of 131I therapy. In comparison, the average time interval of 42 d between FDG PET examinations is quite negligible.
There was no size change in the FDG uptake pattern observed under TSH suppression and TSH stimulation. Sequential examinations with morphologic imaging techniques were not available.
Determination of absolute counting rates in all tumor and background ROIs yielded a significant decrease in FDG uptake in background tissue together with an insignificant increase in FDG uptake in tumor tissue under conditions of TSH stimulation. However, consideration of both parameters led to a significant net increase in the TBR (P < 0.001).
Both the general metabolism and the FDG metabolism of differentiated thyroid carcinoma cells are subject to a complex regulatory system. For example, it is known that the growth and invasion of malignant thyroid cells are regulated not only by TSH but also by a network of mutually influential factors. A significant correlation exists between tumor progression and anomalies in this network (13). Most (13,14), although not all (15), research groups consider TSH to be the classical stimulator of thyroid cell function. In clinical practice, administration of TSH-suppressive dosages of thyroxine is associated with a decreased risk of disease recurrence (16). Studies by Hoelting et al. (17) on follicular cell lines derived from primary tumor, lymph nodes, and distant metastases of the same patient showed first a stimulating (to concentrations ≤100 μU/mL) effect and then an inhibitory effect of TSH on cell growth. The same biphasic response to TSH stimulus has also been shown for the invasive growth potency of tumor cells. In general, cell lines derived from metastases showed a more aggressive pattern of behavior than did cells from the primary tumor. In follicular tumor cell lines, individual cells can be isolated that are not dependent on TSH for their growth yet still synthesize hTg and fulfill other thyroid functions. This variability correlates with the clinically heterogeneous behavior of various tumor manifestations. TSH, on the other hand, seems to positively affect the degree of differentiation of tumor cells. Transfection of human TSH-receptor DNA to primarily receptor-negative cells resulted in a decelerated growth tendency and also showed morphologic signs of a higher grade of differentiation (18,19).
The uptake mechanisms for FDG in tumor cells are complex and are affected by several different factors. The increased glucose metabolism of neoplastic cells was first described by Wartburg (20) and is prerequisite for tumor detection using FDG. Transformed cells show a significantly higher glucose influx than do normal cells. The membranes of such cells often contain glucose transport systems of the GLUT group, which show a higher affinity for glucose normally found only in erythrocytes and brain tissue (21). The high affinity for glucose uptake correlates with the high glycolytic activity of tumor cells. Overexpression of GLUT-1 or GLUT-3 isoforms has been described for various tumor entities (22–24). Haber et al. (25) found no GLUT-1 expression in either normal thyroid tissue or benign adenomas; in up to 50% of all differentiated thyroid carcinomas, there is a pronounced expression of this glucose transport system.
Physiologic studies have shown that the expression of GLUT transporters is affected by TSH (26); furthermore, TSH has been shown to influence glucose metabolism in thyroid cells by stimulating the glycolytic pathway (27). In vitro studies by Filetti et al. (28) showed a significant, dose-dependent increased influx of 2-deoxy-d-glucose in thyroid cells: The data indicate that TSH stimulates the glucose transport system by enhancing the number of functional GLUT transporters in the plasma membrane.
The observation that TSH stimulation was associated with a decreased FDG uptake in the background tissue may be explained by general metabolic changes associated with hypothyroidism. Thus, a diminished net flux and reduced oxidative metabolism of glucose is found in humans (29). The concentrations of gluconeogenic precursors as well as insulin secretion are decreased in the hypothyroid state. The rapid decline in energy reserves of hypothyroid muscle has been attributed to reduced mitochondrial activity (30). McDaniel et al. (31) emphasized that changes in glucose metabolism are multifactorial, including impaired glycogenolysis and gluconeogenesis. Wolf et al. (32) evaluated changes of basal energy expenditure in thyroidectomized patients and found that this expenditure was significantly lower (−15%) in patients while off thyroid hormone therapy compared with a TSH-suppressive state.
FDG, of course, is no pathognomonic substrate for malignant cells. Kubota et al. (33) compared autoradiographic accumulation patterns of 14C-deoxy-d-glucose and histopathologic findings in thyroid cancer xenografts. They found that the uptake of 14C-deoxy-d-glucose was higher in the granulation tissues surrounding necrosis than in viable tumor cells, reflecting not only tumor cell viability and proliferation but also inflammatory and degenerative reactions.
CONCLUSION
FDG PET has been shown to be a sensitive technique to detect iodine-negative tumor manifestations in thyroid cancer. In addition, our findings suggest that FDG uptake in recurrent and metastatic differentiated thyroid carcinomas is dependent on the TSH level and may influence the sensitivity of lesion detection. For clinical use, we recommend that FDG PET examinations in patients with iodine-negative scintigraphy and elevated hTg levels be performed under conditions of TSH stimulation. Follow-up examinations should be conducted under identical TSH conditions and stable study parameters to avoid serious misinterpretations.
Footnotes
Received Aug. 12, 1999; revision accepted Dec. 29, 1999.
For correspondence or reprints contact: Florian Moog, MD, Department of Nuclear Medicine, Ludwig-Maximilians-Universität München, Marchionini-Strasse 15, D-81366 München, Germany.