Abstract
Osteosarcoma is the most common type of primary malignant bone tumor. 18F-FDG PET/CT is useful for staging, detecting recurrence, monitoring response to neoadjuvant chemotherapy, and predicting prognosis. Here, we review the clinical aspects of osteosarcoma management and assess the role of 18F-FDG PET/CT, in particular with regard to pediatric and young adult patients.
- osteosarcoma
- 18F-FDG PET
- clinical outcome
- prediction
- staging
Osteosarcoma is the most common type of primary malignant bone tumor. 18F-FDG PET/CT is useful for staging, detecting recurrence, monitoring response to neoadjuvant chemotherapy (NCT), and predicting prognosis. Here, we review the clinical aspects of osteosarcoma management and assess the role of 18F-FDG PET/CT, in particular with regard to pediatric and young adult patients.
DEMOGRAPHICS
Osteosarcoma is the most common type of malignant primary bone tumor, with a peak incidence in 10- to 14-y-olds (1) and a second peak incidence in those older than 50 y. Approximately 4.4 cases per million are diagnosed annually in people aged 0–24 y (2). Primary osteosarcomas commonly arise within the metaphyses of long bones, with 80% presenting within an extremity. Patients typically present with pain that progresses over weeks to months, with localized swelling and a diminished range of motion or function in the affected limb (3). At the time of diagnosis, approximately 10%–20% of patients have evidence of macroscopic metastasis, most commonly in the lung (81%), bone (34%), and, rarely, lymph nodes (2%) (4). Five-year event-free survival rates are approximately 70% (5). 18F-FDG PET/CT has multiple roles in the evaluation of osteosarcoma for staging, clinical and histologic response to therapy during and at the conclusion of treatment, and determining prognosis after completion of therapy (Fig. 1).
Role of 18F-FDG PET/CT in staging and outcome prediction in osteosarcoma. CTx = chemotherapy; PFS = progression-free survival.
STAGING
18F-FDG PET/CT is useful for staging, detecting recurrence, and predicting histologic response and prognosis in the investigation of osteosarcoma. Conventional imaging modalities other than 18F-FDG PET/CT for staging osteosarcoma are summarized in Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org). During staging, PET/CT is useful for differential diagnosis of primary bone neoplasms and detecting metastatic lesions (Figs. 2 and 3; images are scaled to SUV units as on the color and intensity bars). Combining PET imaging with CT or MRI provides more accurate information on the staging of osteosarcoma than is possible with PET imaging alone (6,7). Also, whole-body imaging with PET/CT can be helpful in staging because osteosarcoma favors the extremities and can be widely metastatic (8). London et al. retrospectively compared the sensitivity and specificity of PET/CT and plain radiography for detecting malignant lesions, including primary and metastatic disease, in patients with pediatric primary bone tumors. PET/CT was more sensitive than plain radiography (98% vs. 83%) and more specific (97% vs. 78%) (9). Strobel et al. compared the diagnostic accuracy of plain radiography, PET, and PET/CT. They found that PET/CT showed 95% sensitivity and 77% specificity, which were the highest rates among the imaging modalities (10). Quartuccio et al. reported that PET/CT has higher accuracy for detecting bone metastasis than CT or dedicated MRI and detects lung metastasis as well as CT does (11). Franzius et al. found that PET performed better than bone scanning for detecting bone metastasis (12), with Hurley et al. finding that PET/CT was more sensitive but bone scanning was more specific (13). Used together in a lesion-based analysis (14), the sensitivity of PET/CT plus bone scanning was 100%, which is higher than that of PET/CT or bone scanning alone, supporting the use of both modalities together to detect bone metastasis (Table 1). This is because some osteoblastic metastases may not show elevated uptake of 18F-FDG, thus yielding false-negative results on PET/CT but positive results on bone scanning (14). In our experience, false-negative cases are rare, and careful review of the CT bone window in a PET/CT scan could reduce them. Thus, we do not routinely perform bone scans on patients with osteosarcoma.
A 13-y-old boy with right leg pain. (A) 18F-FDG PET CT shows 1-cm nodule with markedly elevated uptake in right lower lobe (SUVmax, 6.7) (18F-FDG PET emission image [top], CT transmission image [middle], and PET/CT image [bottom]). (B) Hypointense signal on coronal T1 MR image shows tumor extending from tibial metaphysis through proximal third of right tibia (arrows). (C) Coronal T2 short-tau inversion recovery MR image shows intermediate signal within intramedullary component of tumor (white arrows), corresponding to hypointense T1 signal, and hyperintensity in soft-tissue component lateral to tibia (yellow arrows). (D) 18F-FDG PET/CT anterior maximum-intensity-projection view shows intense uptake in the proximal aspect of the right distal lower extremity. (E) 18F-FDG PET axial emission image (top), transmission CT bone window (middle), and PET/CT image (bottom) show intense though irregularly increased uptake of 18F-FDG in both bone and soft-tissue components of tumor (SUVmax, 15.™).
A 10-y-old girl with pain in right lower extremity. (A) Anterior maximum-intensity-projection PET image shows primary tumor in proximal right tibia and soft tissue (top arrow; SUVmax, 24.4), unsuspected site of metastatic disease in distal right tibia (middle arrow; SUVmax, 12.9), and unsuspected site of metastatic disease in fourth right metatarsal (bottom arrow; SUVmax, 8.2). (B–D) PET (top), CT (middle), and PET/CT fusion (bottom) images showing the sites indicated in A by top arrow (B), middle arrow (C), and bottom arrow (D). Uptake in ipsilateral popliteal, inguinal femoral, and iliac lymph nodes was considered reactive.
Staging and Diagnosis using 18F-FDG PET/CT vs. Other Imaging Modalities
In a recent metaanalysis, the value of PET/CT in diagnosis and staging was summarized (15). This metaanalysis evaluated 26 studies, including the above-mentioned studies. For detecting primary lesions, PET/CT showed 100% sensitivity in 14 studies. Pooled sensitivity and specificity for detecting lung metastases in 8 studies were 81% (95% CI of 72%–88%) and 94% (95% CI of 89%–97%), respectively. For bone metastases, 6 studies showed a pooled sensitivity of 93% (95% CI of 87%–97%) and a pooled specificity of 97% (95% CI of 96%–98%) (Supplemental Fig. 2). Thus, PET/CT is useful for differential diagnosis of the suspected primary bone lesions and for staging.
TREATMENT OVERVIEW
The therapeutic standard of care involves NCT with 3 or more agents with single-agent activity for approximately 8–10 wk, followed by surgical resection of all detectable disease (including metastases) and postoperative adjuvant chemotherapy for 12–29 wk (16). Limb-salvage surgery is the surgical treatment in 85%–90% of patients with osteosarcoma, providing better functional and cosmetic results than amputation without compromising survival (17).
PREDICTION OF RESPONSE TO NCT
Histologic Response
In 1994, a critical appraisal of osteosarcoma prognostic factors concluded that the histologic response of primary tumors after NCT is the most significant factor in predicting disease-free survival (3,18). Although histologic response is prognostic for outcomes, dose-intensifying NCT to increase rates of tumor necrosis have not yielded superior survival curves (19). Similarly, adding chemotherapeutic agents for patients experiencing a poor response has not improved their event-free survival or overall survival (OS) (19). Several grading systems for assessing the effect of NCT on the primary tumor indicate that having at least 90% tumor necrosis after NCT is a favorable response (Table 2) (20–22).
Histologic Response Grading Systems
Role of 18F-FDG PET/CT
In osteosarcoma, the ability of PET or PET/CT to predict histologic response during or after NCT has been reported. In 1996, Jones et al. first reported using PET to assess response to NCT in 3 patients with osteosarcoma (23), finding that SUV declined after NCT, which reflected the histologic response. In the following studies, tumor-to-background ratio or SUVmax after NCT, as well as tumor-to-background ratio or SUVmax ratio between PET at baseline and after NCT, were found to predict histologic response (23–32). Meanwhile, some studies reported that PET/CT was not predictive of histologic response (Table 3; Supplemental Table 1) (33,34). These studies agree on the following optimal cutoffs of PET parameters: an SUVmax of 2–3 after NCT and a tumor-to-background ratio or SUVmax ratio of 0.4–0.6 (24–29,31–33,35–38). In a metaanalysis, an SUVmax after NCT of less than 2.5 and an SUVmax ratio of less than 0.5 between baseline and after NCT were suggested as optimal cutoffs (39). Because of the ability of PET/CT to predict the histologic response, Harrison et al. have proposed further evaluating PET/CT as an imaging biomarker to screen the potential of targeted agents in osteosarcoma (40).
Predicting Histologic Response Using 18F-FDG PET/CT
Predicting the histologic response of tumor during NCT may be a better option than doing so after completion of NCT in the clinic because the latter is too late to modify the NCT regimen. In 2012, Im et al. found that SUVmax after 1 cycle of NCT can also predict the histologic response with an accuracy of 92.9% using a cutoff of 3.2 (37). This result was reproduced by Byun et al. in 2014 (41) and Davis et al. in 2018 (38). Metabolic–volumetric parameters of PET, such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG, the product of tumor segmented SUVmean and MTV), during and after the NCT also showed predictive value for histologic response (36,37,41). Byun et al. reported that SUVmax, MTV, and TLG after 1 cycle of NCT could predict histologic response, but tumor volume assessed by MRI could not (41). Among PET parameters, SUVmax could be a better choice than metabolic–volumetric parameters because MTV and TLG measurements depend on the PET tumor segmentation methods, which are not standardized (42). Also, SUVmax during NCT performed similarly to MTV and TLG in predicting the histologic response (area under the curve, 0.956 for all parameters) (37). Because the level of water molecule diffusion can be changed according to the cellularity of the primary lesion, diffusion-weighted MRI can be used to predict the histologic response of osteosarcoma; thus, combining PET and diffusion-weighted MRI may be an effective method to predict the histologic response (35).
Furthermore, predicting tumor histologic response using the baseline PET/CT has been attempted. Byun et al. used dual-phase (i.e., dual-time point) baseline PET/CT obtained at 60 and 120 min after 18F-FDG injection (43), finding that the mean retention index at baseline PET/CT was predictive of histologic response, with a moderate accuracy of 71% (Supplemental Fig. 3). However, Im et al. reported that although conventional parameters of baseline PET/CT could not predict histologic response, kurtosis and skewness among histogram-based parameters could, with areas under the curve of 0.718 and 0.714, respectively. The predictive value could be enhanced using a machine learning algorithm (area under the curve, 0.821), but the predictive values of texture features depended on the type of segmentation method and machine learning algorithm (44). Altogether, more evidence is needed to prove that baseline PET/CT is predictive of histologic response after NCT.
PREDICTION OF OUTCOMES
Prognostic Factors
Tumor site, size, primary metastases, response to chemotherapy, and surgical remission are the reported independent prognostic factors in osteosarcoma (3). Osteosarcoma arising in pelvic or axial bones has a worse prognosis than that of disease in extremity bones, with pelvic osteosarcoma having reported 5-y OS rates of 27%–47% (45). Osteosarcoma arising in the spine has an even worse prognosis, with a median survival time of 10–23 mo (46). Tumor stage is also prognostic. In the Musculoskeletal Tumor Society’s Enneking system, stage IA showed an almost 100% 5-y survival rate, yet IIB showed a 5-y survival rate of only 40% (47). Among patients with metastases, those with only lung metastases have a more favorable prognosis than do those with extrapulmonary lesions (5-y OS rate, 38% vs. 10.9%) (3). The presence of skip metastasis is associated with a poor prognosis (48). Being younger than 40 y is a good prognostic factor (5-y survival rate, 65.1% vs. 55.0%) (3). In the literature, the most reliable cutoff differentiating favorable from unfavorable response to chemotherapy is 90% necrosis, with greater than 90% necrosis considered favorable (31). Prognostic factors can be applied differently according to age. Response to chemotherapy is applied as a prognostic factor in pediatric patients but not in adult patients (49). Meanwhile, pathologic fracture was associated with poor prognosis in adult patients but not in pediatric patients (50).
Role of 18F-FDG PET/CT
Evaluations of the prognostic value of PET/CT parameters are summarized in Table 4. In 2009, Costelloe et al. was the first to report that SUVmax and TLG, both at baseline and after NCT, could predict progression-free survival and OS (51). Hawkins et al. reported that an SUVmax of over 2.5 after NCT is associated with worse progression-free survival (33). Byun et al. also reported that baseline MTV and TLG could predict metastasis-free survival (52). However, Bailly et al.’s tests of multiple baseline PET/CT parameters for predicting prognosis indicated that only the elongation feature, a type of shape feature, was significantly associated with progression-free survival and OS (53). The elongation feature is the ratio of the longer and shorter edges of the smallest rectangle that encloses the measured region. An elongation factor of 1 indicates maximum symmetry. Most PET/CT parameters were not prognostic in the study, and the parameters were used as continuous variables only. However, the previous studies found that the dichotomized PET/CT parameters are prognostic (53). We reported that most of the dichotomized PET/CT parameters, including SUVmax, SUVpeak, MTV, and TLG at baseline, during, and after NCT, are predictive of event-free survival and OS (Supplemental Fig. 4). Furthermore, we found that the parameters were predictive of event-free survival and OS even after adjustment for the histologic response and initial staging (54).
Predicting Prognosis Via 18F-FDG PET/CT
The studies showed similar results via diverse methods. For example, the segmentation methods of MTV and TLG vary widely. Costelloe et al. set a relative SUV threshold of 45% (51), but Byun et al. used fixed SUV thresholds of 2%, 2.5%, and 45% (52). Im et al. used the fixed SUV thresholds of 2% and 2.5%, relative SUV thresholds of 40% and 60%, and liver-based thresholds, reporting that fixed thresholds or liver-based thresholds were more robust than relative thresholds in predicting prognosis. The MTV and TLG based on relative thresholds were not prognostic during or after NCT because the tumor volumes tend to be overestimated (54). Also, the cutoffs of parameters for predicting prognosis differed: Hawkins et al. used the an SUVmax cutoff of 2.5 (33); the other studies used the optimized cutoffs (51,52,54). All studies were retrospective in some way, necessitating larger, prospective studies to confirm these results and to define the optimal cutoff for predicting prognosis in osteosarcoma.
RECURRENT DISEASE
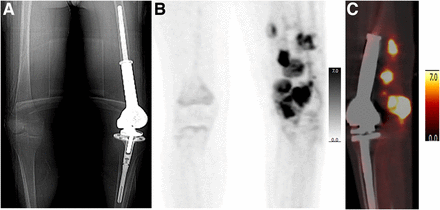
PET/CT can detect osteosarcoma recurrence after completion of treatment (Fig. 4; Supplemental Fig. 5). A metaanalysis of the detection of recurrent disease in 7 studies revealed excellent diagnostic performance, with pooled sensitivity and specificity rates of 91% (95% CI, 81% to 96%) and 93% (95% CI, 87% to 97%), respectively (15). Angelini et al. assessed the diagnostic accuracy of PET/CT for detecting recurrence in 37 patients who were treated with adequate surgical resection, were suspected to have relapsed disease, and had histologic validation of disease relapse after PET/CT. Altogether, 33 patients (89.2%) had PET/CT-detected recurrence. The sensitivity, specificity, and accuracy were 91%, 75%, and 89%, respectively (55). Likewise, Sharp et al. reported that PET/CT was positive in 10 local recurrences, observing either a solid or a peripheral/nodular pattern with a wide range of SUVmax (3.0–15.7) (56). Osteosarcoma has the potential to metastasize to various organs; examples of such metastases include cerebral (Fig. 5), pulmonary pleural caking (Supplemental Fig. 6), epimyocardial (Supplemental Fig. 7), renal (Fig. 6), pancreatic (Supplemental Fig. 8), and tumor thrombosis (Supplemental Fig. 9).
A 15-y-old boy with history of osteosarcoma in left femur and limb-sparing procedure on left lower extremity. (A) Topogram from attenuation-correction CT shows rotating-hinge modular prosthesis in left distal femur and left knee. (B) Anterior maximum-intensity projections shows multiple areas of markedly elevated uptake in left lower extremity. (C) PET/CT sagittal view of left leg shows numerous soft-tissue nodules with intense uptake posterior to prosthesis (SUVmax, 20.3).
A 23-y-old man with progressive metastatic osteosarcoma: attenuation-correction CT image (A), PET emission image (B), PET/CT image (C), and axial T1 MR image (D). 18F-FDG PET/CT shows previously unknown left temporal lobe metastasis (arrows) (SUVmax, 6.4). There is uptake at periphery of lesion, with reduced uptake centrally, consistent with necrosis. Contrast-enhanced T1 MR image obtained following day shows irregularly rimmed ring enhancing lesion in left temporal lobe, consistent with metastatic disease.
A 25-y-old man with multiple recurrent osteosarcoma and large right renal metastasis (arrows; SUVmax, 7.1): anterior maximum-intensity-projection view (A); 18F-FDG PET axial emission image (B), corresponding axial CT image from attenuation correction lesion localization CT (C), and PET/CT image (D).
PET/MRI
Generally, PET/MRI has advantages over PET/CT for its excellent soft-tissue contrast and lower radiation exposure, which is a clear benefit for pediatric patients (57,58). Schäfer reported that PET/MRI showed a 73% reduction in radiation exposure compared with PET/CT and demonstrated an identical rate of detecting lesions (59). Platzek et al. reported that TNM staging of PET/MRI was almost identical to that of conventional modalities (CT plus MRI) in 29 patients with sarcoma (60). Also, Eiber et al. compared the ability of PET/MRI and PET/CT to detect bone metastasis in patients with various primary malignancies. They reported that PET with T1-weighted turbo spin echo MRI did not provide a significant difference in detection of malignant bone lesions but was superior to PET/CT in the anatomic delineation of 18F-FDG-positive lesions (61). Buchbender et al. suggested in their continuing education paper that PET/MRI could provide similar diagnostic accuracy for T staging with MRI alone and similar performance for N staging with PET/CT. (62). Orsatti et al. found that in 13 pediatric sarcoma patients, there was a significant negative correlation between apparent diffusion coefficients and SUV in primary lesions as measured using PET/MRI (63). In response monitoring, whole-body diffusion-weighted MRI during induction chemotherapy could predict clinical response well (area under the curve, 0.98) and exhibited significant agreement with PET/MRI in 56 patients with sarcoma or lymphoma. The biologic and clinical significance of discordant response assessment between diffusion-weighted MRI and PET/MRI in 8 of the 56 patients has not been assessed (64). Therefore, it is warranted to evaluate the additive value by combination of PET/MRI with diffusion-weighted MRI in response monitoring. In addition, Baratto et al. described that PET/MRI can improve monitoring of response to immunotherapy and potentially be used for identification of nonresponders by tumor-associated macrophage imaging using iron oxide nanoparticles (65).
NEW TRACERS
18F-NaF is an excellent bone-seeking agent; therefore, NaF PET has been used for skeletal imaging for many decades (66). Pathologic bone changes and soft-tissue metastasis with dystrophic calcification can be detected by NaF PET (67). Cai et al. reported that NaF PET images could detect hepatic metastasis in patients with osteosarcoma. 18F-NaF activity was more prominent in the calcified lateral portion (68). Chou et al. reported that 18F-NaF could detect cardiac osteosarcoma metastasis, which is a rare metastatic site in patients with osteosarcoma (67). Also, Verma et al. reported that NaF PET could detect a tumor thrombus arising from osteosarcoma (69). Recently, Kairemo et al. proposed NaF PET-based response criteria: NaF PET response criteria for solid tumors (NAFCIST). In the study, the treatment response of 17 patients with metastatic osteosarcoma who were treated with 223RaCl2 was assessed using conventional PERCIST based on 18F-FDG PET/CT and NAFCIST based on NaF PET. NAFCIST could predict the OS but PERCIST could not (70). Altogether, NaF PET is excellent for finding specific tumor regions, including rare metastasis, and could be a good prognostic biomarker.
Radiolabeled fibroblast activation protein inhibitors (FAPIs) have attracted increasing attention as new theranostic agents that specifically target cancer-associated fibroblast (71–73). Kratochwil et al. retrospectively evaluated 68Ga-FAPI PET uptake in 80 patients with 28 different types of malignancies, including 8 patients with sarcoma. The SUVmean of the sarcoma group was the second highest among 28 types of malignancies (71). Koerber et al. retrospectively evaluated the diagnostic ability of 68Ga-FAPI PET in 15 patients with sarcoma, including 1 with osteosarcoma. 68Ga-FAPI PET showed high potential as a probe for diagnosis in sarcoma because it showed high uptake in the primary tumor (median, 7.16; range, 4.64–9.79) (72). Kessler et al. compared the accuracy of 68Ga-FAPI PET with that of 18F-FDG PET in 47 patients with sarcoma, including 8 patients with osteosarcoma. They found a significant association between FAP expression and 68Ga-FAPI PET uptake through histopathologic verification (Spearman r = 0.43, P = 0.03). In per-patient basis analysis, the detection rate of 68Ga-FAPI and 18F-FDG PET were 76.6% and 81.4%, respectively. However, 68Ga-FAPI PET could detect more metastatic lesions, resulting in an upstaging compared with 18F-FDG PET in 8 (18.6%) patients (73). On the basis of the promising results in these early imaging studies of FAPI PET in patients with sarcoma, further studies are warranted to compare the performance of FAPI PET in various clinical situations, such as staging, treatment monitoring, and recurrence, with that of current standard imaging modalities, including 18F-FDG PET. Furthermore, a theranostic approach using FAPI could open new avenues for osteosarcoma management (74).
The membrane glycolipid GD2 is overexpressed in osteosarcoma; however, anti-GD2 immunotherapy for recurrent osteosarcoma has been limited because of the intertumoral heterogeneity of GD2 expression in osteosarcoma. Therefore, immuno-PET agents to assess GD2 expression have been developed and have shown promising results in a preclinical PET/CT study (75) and a PET/MRI case report of an osteosarcoma patient with lung metastasis (76).
CONCLUSION
In osteosarcoma, 18F-FDG PET/CT is useful for staging, monitoring response to therapy, predicting prognosis, and characterizing recurrent disease. It has been extensively validated in studies involving pediatric patients, and similar findings have also been observed in research that includes adult patients, highlighting its effectiveness across different age groups. 18F-FDG PET/CT could ease the drug development process for newer osteosarcoma therapies by facilitating patient selection based on prognosis and serving as early determinants of disease response.
ACKNOWLEDGMENTS
We thank Cherise Guess, PhD, ELS, senior scientific editor, St. Jude Children’s Hospital, for expert review and advice, and Amanda Meek for expert administrative support.
Footnotes
Learning Objectives: On successful completion of this activity, participants should be able to describe (1) the diagnosis and treatment strategy and response criteria of osteosarcoma; (2) the roles of 18F-FDG PET in staging, prediction of clinical outcome and detection of recurrent disease; and (3) the useful timepoint of 18F-FDG PET in prediction of clinical outcome.
Financial Disclosure: Drs. Chiwoo Oh and Hyung-Jun Im were supported by National Research Foundation of Korea (NRF) (NRF-2021M2E8A1039564, 2020R1C1C1009000, and 2019M2D2A1A01058210), Korea Evaluation Institute of Industrial Technology (KEIT) grant funded by the Korea government (MOTIE) (no. 20018522), and Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (HN22C0632). Dr. Shulkin was supported by the American Lebanese Syrian Associated Charities (ALSAC). Dr. Im is the cofounder and the Chief Scientific Officer of Portrai. The authors of this article have indicated no other relevant relationships that could be perceived as a real or apparent conflict of interest.
CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, SAM, and other credit types, participants can access this activity through the SNMMI website (http://www.snmmilearningcenter.org) throsugh June 2026.
Published online May. 18, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.
- 41.
- 42.
- 43.
- 44.
- 45.
- 46.
- 47.
- 48.
- 49.
- 50.
- 51.
- 52.
- 53.
- 54.
- 55.
- 56.
- 57.
- 58.
- 59.
- 60.
- 61.
- 62.
- 63.
- 64.
- 65.
- 66.
- 67.
- 68.
- 69.
- 70.
- 71.
- 72.
- 73.
- 74.
- 75.
- 76.
- Received for publication February 15, 2023.
- Revision received April 26, 2023.