Abstract
We demonstrated increased 18F-FDG uptake in injured peripheral nerves in a model of neuropathic pain using small-animal PET/MRI. Methods: A neuropathic pain model in rats was created by spared-nerve injury of the left sciatic nerve. Sham-operated rats without nerve injury were used as a control. The presence of pain was confirmed by testing for allodynia. Sequential small-animal 18F-FDG PET and MRI scans of the thighs were obtained and coregistered. Autoradiography was performed on harvested nerves and muscle. Results: The group with spared-nerve injury showed the development of allodynia in the operated limb (P < 0.001). Increased 18F-FDG uptake was observed on both PET/MRI (P < 0.001) and autoradiography (P < 0.005) in the operated nerve in this group. 18F-FDG uptake in the nerves correlated well with allodynia (ρ = −0.59; P < 0.024). Conclusion: Animals with neuropathic pain show increased 18F-FDG uptake in the affected nerve. Small-animal PET/MRI can be effectively used to localize 18F-FDG uptake in peripheral nerves.
- PET/MRI
- neuropathic pain
- 18F-FDG
- spared nerve injury
Increased spontaneous activity (1) and metabolic changes (2,3) are known to occur in injured nerves and are largely attributed to the symptoms of neuropathic pain. Neuronal activity is dependent on glucose metabolism (4). Isolated cases of increased 18F-FDG uptake in peripheral nerves have been reported in noncancerous cases of peripheral neuropathy and neural inflammation (5,6). Little is known about glucose metabolism in the peripheral nervous system using 18F-FDG in the setting of noncancerous nerve injury. To our knowledge, this study was the first systematic study of 18F-FDG uptake in the peripheral nerves in a noncancerous setting, specifically in a rat model of neuropathic pain. Furthermore, we demonstrated the utility of coregistered MRI in localizing metabolic activity in peripheral nerves.
MATERIALS AND METHODS
Neuropathic Pain Model
Animal experiments were approved by the institutional animal care and use committee. Two groups of male adult Sprague–Dawley rats weighing 200–250 g were used. The first was the spared-nerve injury group (n = 3), which underwent a left spared-nerve injury procedure that creates a well-characterized neuropathic pain model (7). Briefly, under aseptic surgical techniques and inhalational 2%–3% isoflurane anesthesia, the left sciatic nerve and its 3 terminal branches were surgically accessed. A ligation and axotomy of the tibial and common peroneal nerves was performed, sparing the sural nerve. The second group was a control group (n = 3), which was subjected to a sham operation similar to that used on the spared-nerve injury animals but without the nerve injury. Animals were permitted to heal for 4 wk after the surgery.
Pain Behavior Assessment
Allodynia, a common feature of neuropathic pain, is pain that results from a stimulus that would normally not provoke pain. Development of mechanical allodynia was evaluated during the third week after surgery using von Frey hair filaments. Animals were acclimatized on a raised wire-mesh platform for 2 h/d for 4 d before testing and an hour just before testing. The filament was applied to the lateral portion of the plantar aspect of both hind paws through the mesh floor and pressed until it bent, and then kept in place for 8 s. A brisk withdrawal of the paw was considered a positive response, which was confirmed with the same filament again after at least 60 s. The testing endpoint was 3 consecutive positive responses for the same filament or if the filament lifted the paw off the floor. The data thus collected were analyzed using the PsychoFit software program (http://psych.colorado.edu/∼lharvey/html/software.html), which calculates the 50% withdrawal threshold in log filament stiffness units. The threshold is defined as the stimulus intensity at which the withdrawal is detected 50% of the time (8,9).
Small-Animal PET/MRI
All animals were anesthetized with humidified 2%–3% isoflurane throughout imaging. Longitudinal fiducial markers containing dilute tracer solution (1.3 MBq/mL) were fixed to the animal holder. The animal was rigidly secured in a transportable holder to minimize motion while being transferred between the PET and MRI scanners. The animals underwent sequential small-animal PET (microPET R4; Siemens Medical Solutions) and small-animal MRI (a self-shielded 30-cm-bore 7-T magnet [Varian] with a 9-cm-bore gradient insert [Resonance Research Inc.] using EXCITE2 electronics and the supporting LX11 platform; GE Healthcare]. For the PET scan, 18.5 MBq of 18F-FDG were injected intravenously and the animals were kept anesthetized on a warmed platform (35°C) for 1 h before imaging. A 10-min static scan of the thighs was obtained, after which the animals were transferred to the MRI scanner. T1-weighted fast spin-echo images (repetition time, 800 ms; echo time, 7.7 ms; slice thickness, 1 mm; in-plane resolution, 234 μm2) were obtained of the thighs in an ambient temperature maintained at 35°C.
Autoradiography
Immediately after undergoing PET/MRI, the animals were sacrificed, and the sciatic nerves and adjacent muscles were harvested. The tissues were placed on a phosphor screen (medium MultiSensitive Phosphor Screen; PerkinElmer) and exposed for 24 h. The screen was imaged using a variable-mode imager (Typhoon 9410; GE Healthcare).
Image Analysis
The PET and MRI scans were coregistered using AMIDE image analysis software (bin 0.9.2; http://amide.sourceforge.net). The MRI images were used to define the anatomic location of the peripheral nerves and the placement of regions of interest (ROIs). Radioactivity counts were then recorded from within the ROIs in the fused PET images. The average signal from ROIs in adjacent muscle was used to normalize the maximum signal from the nerve ROIs, which were placed on 5-mm segments of sciatic nerve in each thigh, proximal to the level of injury.
Raw autoradiography images were converted to tagged image file format and analyzed using OsiriX imaging software (version 3.7.1; http://www.osirix-viewer.com/index.html). Background-subtracted maximum signal from ROIs placed around entire nerves was normalized to background-subtracted mean signal from ROIs placed within muscles.
Statistical Analysis
To test the ability to detect changes in the spared-nerve injury group, nonparametric marginal models (10) were estimated and the group × side interaction (or, in the case of the von Frey results, the group × side × time interaction) was tested for significance. A Bonferroni-adjusted 1-sided P value of 0.0125 was used as the significance level. Statistical analyses were done with R software (version 2.9.2; www.r-project.org), using version 1.2 of the “nparLD” package. Results are expressed as mean ± SD. Correlations among withdrawal thresholds by von Frey tests, 18F-FDG signal with PET/MRI, and 18F-FDG signal with autoradiography were calculated by Spearman rank-order correlations, and exact tests were done to assess their statistical significance using a 1-sided P value of 0.05. A single tie in von Frey scores was broken in favor of the null hypothesis.
RESULTS
Animals with Spared-Nerve Injury Exhibit Allodynia
Development of allodynia, assessed using the von Frey hair test, was seen only in the spared-nerve injury group (Table 1). Lower withdrawal thresholds were seen in the left hind limb of the spared-nerve injury group after surgery than before surgery (in log stiffness units, 5.38 ± 0.24 at baseline and 4.72 ± 0.16 after surgery; P < 0.001). No change in withdrawal thresholds was observed after surgery in the right hind limb of the spared-nerve injury group or in either hind limb of the control group.
Withdrawal Threshold Data for Right and Left Hind Paws Before and After Surgery
Sciatic Nerves with Spared-Nerve Injury Show Increased 18F-FDG Uptake on PET/MRI
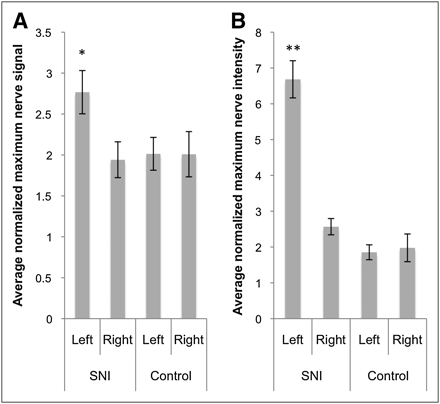
On the coregistered PET/MRI scans, increased tracer uptake was visualized in the injured sciatic nerve in animals with spared-nerve injury (Fig. 1A). Significantly higher normalized maximum 18F-FDG uptake was found in the left sciatic nerve of the spared-nerve injury group (2.77 ± 0.46, P < 0.001) than in the right sciatic nerve of that group (1.94 ± 0.38) or in either sciatic nerve of the control group (left, 2.01 ± 0.35; right, 2.01 ± 0.48) (Fig. 2A).
Representative spared-nerve injury (top row) and control (bottom row) animals showing sciatic nerves (arrows) on transaxial MRI, PET, and PET/MRI (A) and autoradiography of excised sciatic nerves (B). On 18F-FDG PET/MRI, significantly increased normalized 18F-FDG uptake is seen on side with spared-nerve injury (left), compared with control side (right). Control animals did not show any significant difference between right and left nerves. Autoradiography of sciatic nerve specimens from spared-nerve injury animals showed that normalized radiotracer uptake is higher in injured sciatic nerve (left) than in control sciatic nerve (right). Control animals did not show any significant difference between right and left nerves. SNI = spared-nerve injury.
Average normalized maximum signal in sciatic nerves on PET/MRI (A) and autoradiography (B). Error bars represent SE of mean. On both PET/MRI and autoradiography, greater 18F-FDG uptake is seen in left nerve of spared-nerve injury group than in right nerve of spared-nerve injury group or in either nerve of control group. SNI = spared-nerve injury. *P < 0.001. **P < 0.005.
Sciatic Nerves with Spared-Nerve Injury Show Increased 18F-FDG Uptake on Autoradiography
After PET/MRI, both sciatic nerves were harvested from each animal for autoradiography. Significantly increased normalized maximum 18F-FDG uptake was seen in the left sciatic nerve of the spared-nerve injury group, especially in the neuroma at the site of transection (6.68 ± 0.90, P < 0.005) (Fig. 1B), compared with the right sciatic nerve of that group (2.57 ± 0.39) or either sciatic nerve of the control group (left, 1.85 ± 0.36; right, 1.98 ± 0.67) (Fig. 2B).
Correlation of 18F-FDG Uptake Is Observed in Nerves and Pain Behavior
A good correlation was observed between 18F-FDG uptake on PET/MRI (normalized maximum signal in left and right nerves) and behavioral measurements (withdrawal threshold in right and left hind paws) of spared-nerve injury and control animals (ρ = −0.59, n = 12, P = 0.024). Lower withdrawal thresholds (increased pain sensitivity) correlated with higher normalized 18F-FDG uptake in the nerves. A similar negative correlation, which was not significant, was seen between 18F-FDG uptake on autoradiography and behavioral measurements (ρ = −0.42, n = 12, P = 0.088). 18F-FDG uptake on PET/MRI and autoradiography was well correlated (ρ = 0.72, n = 12, P = 0.006) (Fig. 3).
Scatterplots between withdrawal thresholds on von Frey testing, normalized 18F-FDG uptake on PET/MRI, and normalized 18F-FDG uptake on autoradiography as seen on left and right sides of spared-nerve injury and control groups. SNI = spared-nerve injury.
Increased 18F-FDG Uptake Is Observed in Ipsilateral Calf Tissues of Spared-Nerve Injury Animals
Interestingly, significantly increased soft-tissue uptake of 18F-FDG was seen in the calf tissues of spared-nerve injury animals on the same side as the injured nerve (Fig. 4). Because we had not anticipated such an observation, we did not include the distal parts of the hind limbs in the MRI scans. Cylindric ROIs (3 × 5 mm) were placed on the calf, carefully avoiding the tibiae, in all PET images. The mean signal in these ROIs was normalized to the mean muscle signal in the thigh muscles. Increased 18F-FDG uptake was seen in the calf soft tissues on the side ipsilateral to the nerve injury in the spared-nerve injury group (5.59 ± 1.62, P < 0.001), compared with the contralateral calf in the spared-nerve injury group (1.95 ± 0.67) or either calf in the control group (left, 1.75 ± 0.31; right, 1.89 ± 0.38).
Increased soft-tissue uptake of 18F-FDG in ipsilateral calf of spared-nerve injury animals. PET images were acquired of representative spared-nerve injury animal through its hind limbs: transaxial section (A), coronal section (B), sagittal section through right hind limb (C), and sagittal section through left hind limb (D). Arrows point to soft tissue with visibly increased 18F-FDG uptake in left hind limb.
DISCUSSION
Increased cellular metabolism and glucose utilization can be observed in injured nerves. These observations may be due to increased spontaneous firing in pain-sensing neurons (2,3,11) and may be part of a neuronal response to nerve injury, including neuronal stress and nerve regenerative processes such as axonal sprouting and protein synthesis (12). 18F-FDG is a well-established metabolic activity marker using PET and, by extension, can be considered a marker for increased neuronal activity in the setting of nerve injury and neuropathic pain.
18F-FDG has already been used to study neuronal metabolic activity changes in the cerebrum (13–15) and specifically in relation to chronic pain (16). To our knowledge, this study was the first to address nociception-related 18F-FDG uptake in peripheral nerves. Metabolic imaging of peripheral nociception has been challenged by the relatively poor spatial resolution of small-animal PET, which varies from 0.7 to 1.84 mm in full width at half maximum in phantom studies (17) (the sciatic nerve is 1–2 mm thick in rats), and the difficulty in distinguishing peripheral nerves from background activity using PET alone.
Because of their better spatial resolution, CT and MRI have been established as anatomic localization tools with PET. By coregistering images from 2 modalities, we were able to use the excellent spatial and contrast resolution of MRI to locate sciatic nerves and then use 18F-FDG PET to observe metabolic changes in them associated with nerve injury.
In this study, increased 18F-FDG uptake was seen in neuropathic nerves. The radiotracer uptake detected with PET/MRI correlated well with that seen on autoradiography, both of which also correlated well with pain behaviors. Although the correlations were driven by the small number of pain responses (Fig. 3), they demonstrated the mutual consistency of 18F-FDG uptake measurements in the nerves by PET/MRI and autoradiography.
Incidentally, increased 18F-FDG was seen in the gastrocnemius muscles of the calf on the side of nerve injury in spared-nerve injury animals (Fig. 4). On reviewing the literature for an explanation, we found that Handberg et al. reported, in sciatic nerve transections, an increase in basal glucose transport in the denervated calf muscles (18).
CONCLUSION
PET/MRI can potentially be used as a tool to image and evaluate peripheral neural diseases. Using MRI guidance, subtle changes in tissue metabolism can be appreciated within discrete soft-tissue structures that would not be otherwise possible using PET only or CT. Considering the small sizes and similar densities of various tissues and organs in small animals, small-animal PET/MRI can potentially serve as a valuable preclinical tool.
DISCLOSURE STATEMENT
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr. David Dick and his colleagues for the production of 18F-FDG in the Cyclotron Radiochemistry Facility, and Drs. Laura Pisani, Timothy Doyle, and Aileen Hoehne for assistance with the small-animal imaging devices and autoradiography in the Small Animal Imaging Facility at Stanford University. Support was provided in part by the Weston Havens Foundation and seed funding from the Department of Radiology. No other potential conflict of interest relevant to this article was reported.
- © 2011 by Society of Nuclear Medicine
REFERENCES
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- Received for publication October 28, 2010.
- Accepted for publication April 4, 2011.