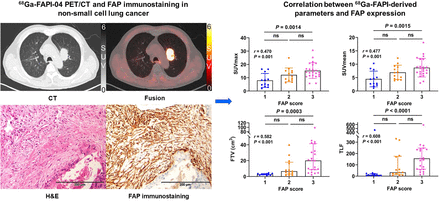
Visual Abstract
Abstract
Fibroblast activation protein (FAP) is a promising diagnostic and therapeutic target in various solid tumors. This study aimed to assess the diagnostic efficiency of 68Ga-labeled FAP inhibitor (FAPI)–04 PET/CT for detecting lymph node metastasis in non–small cell lung cancer (NSCLC) and to investigate the correlation between tumor 68Ga-FAPI-04 uptake and FAP expression. Methods: We retrospectively enrolled 136 participants with suspected or biopsy-confirmed NSCLC who underwent 68Ga-FAPI-04 PET/CT for initial staging. The diagnostic performance of 68Ga-FAPI-04 for the detection of NSCLC was evaluated. The final histopathology or typical imaging features were used as the reference standard. The SUVmax and SUVmean, 68Ga-FAPI–avid tumor volume (FTV), and total lesion FAP expression (TLF) were measured and calculated. FAP immunostaining of tissue specimens was performed. The correlation between 68Ga-FAPI-04 uptake and FAP expression was assessed using the Spearman correlation coefficient. Results: Ninety-one participants (median age, 65 y [interquartile range, 58–70 y]; 69 men) with NSCLC were finally analyzed. In lesion-based analysis, the diagnostic sensitivity and positive predictive value of 68Ga-FAPI-04 PET/CT for detection of the primary tumor were 96.70% (88/91) and 100% (88/88), respectively. In station-based analysis, the diagnostic sensitivity, specificity, and accuracy for the detection of lymph node metastasis were 72.00% (18/25), 93.10% (108/116), and 89.36% (126/141), respectively. Tumor 68Ga-FAPI-04 uptake (SUVmax, SUVmean, FTV, and TLF) correlated positively with FAP expression (r = 0.470, 0.477, 0.582, and 0.608, respectively; all P ≤ 0.001). The volume parameters FTV and TLF correlated strongly with FAP expression in 31 surgical specimens (r = 0.700 and 0.770, respectively; both P < 0.001). Conclusion: 68Ga-FAPI-04 PET/CT had excellent diagnostic efficiency for detecting lymph node metastasis, and 68Ga-FAPI-04 uptake showed a close association with FAP expression in participants with NSCLC.
Lung cancer is the leading cause of cancer-related mortality in men and the second-leading cause in women worldwide, with a 5-y survival rate of only 10%–33% (1). Non–small cell lung cancer (NSCLC) is the most common histologic type and accounts for more than 80% of all lung cancers (2). An accurate definition of disease extent at initial diagnosis is important for informing the choice of treatment strategy and patient management in NSCLC.
PET/CT combining anatomic and functional imaging capabilities can improve the staging accuracy of lung cancer compared with conventional imaging (3); however, 18F-FDG, as the most commonly used imaging agent, has some limitations. Some factors (e.g., lesion diameter < 8–10 mm, mucinous adenocarcinoma, ground-glass nodule) may lead to false-negative findings, whereas reactive lymphoid hyperplasia, lymphadenitis, granulomatous inflammation, and tuberculosis can lead to false-positive findings, resulting in the misdiagnosis of lymph node metastasis (4,5). The identification of novel molecular targets and development of new imaging agents are thus critical for lung cancer evaluation (6,7).
Cancer-associated fibroblasts are an important component of the tumor microenvironment, contributing to epithelial cell growth and tumorigenicity and promoting tumor invasion and metastasis (8). Fibroblast activation protein (FAP) is a marker expressed specifically on the surface of cancer-associated fibroblasts. FAP inhibitors (FAPIs), which specifically target FAP, have attracted increasing interest, and various radiopharmaceuticals based on quinoline have recently been developed as pan-cancer tracers with favorable characteristics and good clinical application prospects (9).
Several recent studies investigating the diagnostic efficiency of 68Ga-FAPI and 18F-FAPI PET/CT in lung cancer have achieved initial encouraging results (10–15). 68Ga-FAPI demonstrated good staging efficiency, particularly for the detection of primary tumors and distant metastasis (bone and pleura) (11,12,14); however, the diagnostic efficacy of 68Ga-FAPI for the detection of lymph node metastasis remains uncertain because of limited histologic evidence provided by only 2 studies (12,14). In addition, despite the high detection rate of lung cancer by 68Ga-FAPI PET/CT, its usefulness in NSCLC is also currently limited by a lack of histopathologic evidence regarding tumor FAP expression level, which is crucial for the future routine application of 68Ga-FAPI in clinical practice. We therefore aimed to evaluate the diagnostic performance of 68Ga-FAPI-04 PET/CT for the diagnosis of lymph node metastasis and to investigate the correlation between 68Ga-FAPI-04 uptake by the primary tumor and FAP immunostaining in NSCLC.
MATERIALS AND METHODS
Participants
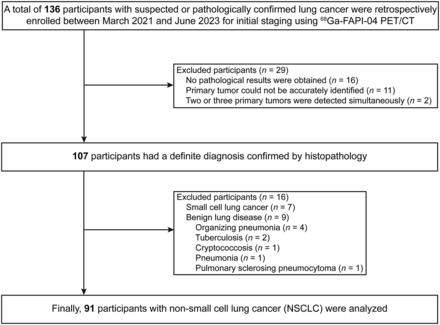
This was a secondary analysis of an ongoing clinical trial at our hospital. The study was approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University and registered in ClinicalTrails.gov (NCT05034146), and each participant signed an informed consent form. Participants with suspected or biopsy-proven NSCLC were enrolled for initial clinical staging from March 2021 to June 2023. The inclusion criteria were suspected or newly diagnosed NSCLC and no antitumor treatment before PET/CT. The exclusion criteria were no definite pathologic diagnosis, 2 or more concurrent primary tumors, small cell lung cancer, and benign lung disease.
Details of image acquisition (16,17), imaging analysis (18), and diagnosis reference standards (19–21) are provided in the supplemental materials (available at http://jnm.snmjournals.org).
Immunostaining of FAP and Glucose Transporter-1 (GLUT-1)
FAP expression levels in all available tissues were confirmed by immunostaining. Paraffin-embedded tissues were cut into 4-μm sections for hematoxylin–eosin, FAP, and GLUT-1 staining. The senior pathologist observed all stained sections under a light microscope (Olympus BX-53). The immunostaining results for FAP and GLUT-1 were scored semiquantitatively on the basis of the intensity and proportion of positive signal in the cytoplasm of stromal fibroblasts and cancer cells, respectively. The final immunostaining scores were 0 (negative), 1 (mild), 2 (intermediate), and 3 (intense) (supplemental materials).
Statistical Analysis
Statistical analysis was performed using SPSS (version 19.0) and GraphPad Prism (version 8). Categoric variables were presented as numbers with percentages, and continuous variables were presented as mean ± SD or as median with interquartile range, according to whether the data were normally distributed. The diagnostic efficiency of 68Ga-FAPI-04 for NSCLC was calculated. Independent sample t tests were used to compare 2 continuous variables with normal distributions; otherwise, the Mann–Whitney U test was used. Diagnostic accuracy was compared using McNemar test. The correlation between FAP expression and 68Ga-FAPI-04 uptake was analyzed using the Spearman correlation coefficient. Multiple groups of data were compared using 1-way ANOVA. The area under the receiver operating characteristic curve was calculated. A 2-tailed P value of less than 0.05 represented statistical significance.
RESULTS
Participant Characteristics
We retrospectively enrolled 136 participants, of whom 91 with NSCLC with a surgery- or biopsy-proven pathologic diagnosis were analyzed. The clinicopathologic characteristics of the participants are summarized in Table 1 and Supplemental Table 1. The participant selection process is shown in Figure 1.
Clinicopathologic Features of Participants with NSCLC
Detailed selection process of participants included in this study.
Primary Tumor Detection
We evaluated 91 primary tumors in 91 participants with NSCLC. The diagnostic sensitivity and positive predictive value of 68Ga-FAPI-04 PET/CT for the detection of primary tumor based on visual evaluation were 96.70% (88/91) and 100% (88/88), respectively. Representative true-positive and false-negative cases are shown in Figure 2.
Represent true-positive (A) and false-negative (B) mixed ground glass nodules on chest CT, 68Ga-FAPI-04 PET/CT, and corresponding hematoxylin–eosin (H&E) and FAP immunostaining (magnification ×100). (A) 76-y-old woman with history of postoperative right-sided breast cancer 1 y previously with 68Ga-FAPI–avid adenocarcinoma (ADC; arrows) measuring 18 × 12 × 15 mm in left upper lobe with moderate FAP expression (pT1bN0M0, IA2). (B) 76-y-old man with ADC (arrows) measuring 13 × 10 × 20 mm in right upper lobe with visually negative 68Ga-FAPI-04 uptake and weak FAP expression (pT1bN0M0, IA2).
The 68Ga-FAPI–avid tumor volume (FTV) and total lesion FAP expression (TLF) of the primary tumor were higher in men and in participants with a smoking history, larger tumor size (>3 cm), higher clinical stage (III–IV), and nonadenocarcinoma. The SUVmax of the primary tumor was higher in participants with larger tumors (>3 cm) (15.10 vs. 8.53, P < 0.001), higher clinical stage (III–IV) (14.86 ± 5.78 vs. 10.06 ± 4.80, P < 0.001), and nonadenocarcinoma (15.15 ± 5.95 vs. 12.23 ± 5.64, P = 0.02). Participants with larger tumors and higher clinical stage also had higher tumor-to-background and tumor-to-liver ratios. The time interval between injection and acquisition had no effect on 68Ga-FAPI-04 uptake in the primary tumor (Supplemental Table 2). The median SUVmax (14.93 vs. 12.75, P = 0.031), FTV (26.88 vs. 4.52, P < 0.001), and TLF (222.77 vs. 30.14, P < 0.001) were higher in squamous cell carcinomas (SCCs) than in adenocarcinomas.
Lymph Node Metastasis Detection
Thirty-one participants underwent surgical lymph node dissection, and 7 received endobronchial ultrasound–guided transbronchial needle aspiration puncture biopsy of the mediastinal lymph nodes. In total, 141 stations comprising 357 lymph nodes were assessed. The diagnostic sensitivity, specificity, and accuracy of 68Ga-FAPI-04 in station-based analysis were 72.00% (18/25), 93.10% (108/116), and 89.36% (126/141), respectively (Table 2), and the equivalent values in lesion-based analysis were 58.06% (18/31), 94.79% (309/326), and 91.60% (327/357), respectively, for the detection of lymph node involvement by 68Ga-FAPI-04 (Supplemental Table 3). The pathologic results demonstrated that false-positive lymph nodes were reactive lymphoid hyperplasia. Representative cases of true-positive and false-positive lymph nodes are shown in Figure 3.
Diagnostic Performance of 68Ga-FAPI-04 PET/CT for Detection of Lymph Node Metastasis in NSCLC (Station-Based Analysis)
Representative cases of true-positive and false-positive lymph nodes on 68Ga-FAPI-04 PET/CT. (A) PET/CT images showed 68Ga-FAPI–avid soft-tissue mass (black and white arrows) and enlarged lymph node (red arrows) in ipsilateral mediastinum (station 5) with intense 68Ga-FAPI-04 accumulation (SUVmax, 7.46) in 54-y-old man. Histopathology demonstrated adenocarcinoma with lymph node metastasis (pT2aN2M0, IIIA). (B) PET/CT images revealed well-defined solid 68Ga-FAPI–avid mass (black arrow) and enlarged lymph nodes in left hilum with intense 68Ga-FAPI-04 uptake (SUVmax, 10.33; red arrows) in 62-y-old man. Histopathology confirmed adenocarcinoma with reactive hyperplasia of left hilar lymph node (pT2bN0M0, IIA).
Metastatic lymph nodes had a larger median short diameter (1.06 vs. 0.56 cm), SUVmax (7.46 vs. 1.82), SUVmean (4.23 vs. 1.23), tumor-to-background ratio (4.01 vs. 0.87), and tumor-to-liver ratio (6.00 vs. 1.44) than nonmetastatic lymph nodes (all P < 0.001) (Supplemental Table 4). 68Ga-FAPI-04 showed excellent predictive efficiency for lymph node involvement, with a diagnostic sensitivity and specificity for differentiating lymph node metastasis of 62.96% (17/27) and 96.70% (176/182), respectively, using an SUVmax of 4.815 as the optimal cutoff (Supplemental Table 5).
Changes in N-Stage, TNM Stage, and Therapeutic Management
Thirty-one participants underwent lymph node dissection, including 23 N0, 4 N1, and 4 N2. 68Ga-FAPI-04 accurately predicted N-stage in 21 participants (67.74%, 21/31), overestimated it in 6, and underestimated it in 4. Compared with 68Ga-FAPI-04, conventional imaging (chest contrast-enhanced CT) accurately determined N-stage in 19 (61.29%, 19/31) participants, overestimated it in 7, and underestimated it in 5 (Supplemental Table 6). The diagnostic accuracy of 68Ga-FAPI-04 was higher than that of conventional imaging, but the difference was not significant (P = 0.727).
The diagnostic accuracies of 68Ga-FAPI-04 and conventional imaging for TNM stage were 82.42% (75/91) and 68.13% (62/91), respectively (P = 0.029). 68Ga-FAPI-04 PET/CT overestimated the stage in 6 participants and underestimated it in 7, and the primary tumor could not be identified because of negative 68Ga-FAPI-04 uptake in 3 participants. TNM stage was misdiagnosed on conventional imaging in 29 participants, including 7 overestimated, 17 underestimated, and 5 primary tumors missed. Compared with conventional imaging, 68Ga-FAPI-04 PET/CT caused a change in treatment management for 21 (23.08%, 21/91) participants; primary tumors were found in 4 participants and the therapeutic regimens were changed in 17 participants (Supplemental Table 7).
Correlation of Tumor Uptake with FAP and GLUT-1 Expression
Forty-eight primary tumor samples (31 surgical and 17 biopsy specimens) were evaluated with an FAP-positive staining rate of 100%. FAP staining was found not only in cancer-associated fibroblasts in the tumor stroma but also in a few tumor cells in 12 cases. 68Ga-FAPI–derived SUVmax, SUVmean, FTV, and TLF correlated positively with FAP expression level in the primary tumor (r = 0.470, 0.477, 0.582, and 0.608, respectively; all P ≤ 0.001) (Fig. 4; Supplemental Fig. 1). For 31 surgical specimens, SUVmax and SUVmean showed moderate positive correlations with tumor FAP expression level (r = 0.614 and 0.624, respectively; both P < 0.001), and FTV and TLF showed strong correlations with tumor FAP expression (r = 0.700 and 0.770, respectively; both P < 0.001). The other 17 biopsy specimens showed no correlation between tumor 68Ga-FAPI-04 uptake and FAP expression. 68Ga-FAPI–derived parameters also showed moderate positive correlations (r = 0.610, 0.623, 0.561, and 0.684, respectively; all P ≤ 0.002) with FAP expression in adenocarcinomas (n = 29). FAP expression levels were generally higher in SCCs (n = 15), with no relationship between tumor 68Ga-FAPI-04 uptake and FAP expression.
Representative 68Ga-FAPI-04 PET/CT, hematoxylin-eosin (H&E), and FAP immunostaining (magnification ×100) images of pulmonary adenocarcinoma (ADC, A) and SCC (B). (A) Top row: 48-y-old woman with ADC (arrows) in right middle lobe with mild 68Ga-FAPI-04 uptake and weak FAP expression (pT1bN0M0, IA2). Middle row: 49-y-old woman with 68Ga-FAPI–avid ADC (arrows) in right inferior lobe with moderate FAP expression (pT2aN0M0, IB). Bottom row: 66-y-old man with ADC (arrows) in left superior lobe with intense 68Ga-FAPI-04 uptake and strong FAP expression (pT1bN0M0, IA2). (B) Top row: 78-y-old man with SCC (arrows) in right superior lobe with intense 68Ga-FAPI-04 uptake and moderate FAP expression (cT3N0M1c, IVB). Bottom row: 66-y-old man with 68Ga-FAPI-04–avid SCC (arrows) in left superior lobe with strong FAP expression (pT2aN1M0, IIB).
Six NSCLC tumor tissues also underwent FAP and GLUT-1 double staining (Fig. 5). FAP was expressed predominantly in the tumor stroma and a small proportion of tumor cells, whereas GLUT-1 was expressed mainly in tumor cells. The median immunostaining scores were 2.50 (interquartile range, 1–3) for FAP and 2 (interquartile range, 0.75–3) for GLUT-1. The 18F-FDG-derived SUVmean correlated significantly with GLUT-1 expression (r = 0.926, P = 0.008) (Supplemental Table 8).
Representative 68Ga-FAPI-04, 18F-FDG PET/CT, and double immunostaining (magnification ×100) images of participants with NSCLC. (A) 67-y-old woman with adenocarcinoma (ADC; arrows) in right lower lobe (cT2N2M0, IIIA). (B) 65-y-old woman with ADC (arrows) in right upper lobe (pT1cN0M0, IA3). (C) 75-y-old man with ADC (arrows) in left lower lobe (ypT1aN0M0). (D) 68-y-old man with SCC (arrows) in left upper lobe (cT4N0M0, IIIA). Brown and rose-red in double immunostaining images represent FAP and GLUT-1 immunostaining, respectively. MTV = metabolic tumor volume; TLG = total lesion glycolysis.
Comparison of Tumor 68Ga-FAPI-04 Uptake Between Different FAP-Immunostaining Scores
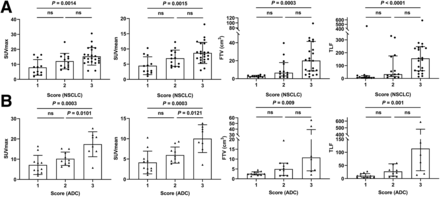
According to the integrated FAP expression scores, 48 participants were finally classified as scores 1 (n = 12), 2 (n = 13), or 3 (n = 23) (Fig. 6). The 68Ga-FAPI–derived SUVmax, SUVmean, FTV, and TLF increased gradually with increasing score (all P < 0.003). Participants with a score of 3 had higher 68Ga-FAPI-04 uptake than those with a score of 1 in terms of mean SUVmax (15.33 ± 5.84 vs. 7.94 ± 5.24), SUVmean (8.70 ± 3.48 vs. 4.50 ± 2.94), median FTV (20.12 vs. 2.57 cm3), and TLF (155.70 vs. 10.69) (all P < 0.002). Among 29 participants with lung adenocarcinoma, a higher FAP-immunostaining score indicated intense tumor 68Ga-FAPI-04 uptake, but there was no significant difference between the groups with scores of 2 (n = 3) and 3 (n = 12) in participants with SCC.
Comparison of 68Ga-FAPI-04 uptake in primary tumor in relation to FAP-immunostaining score in participants with NSCLC (A) and lung adenocarcinoma (ADC, B). Lines in SUVmax and SUVmean groups represent mean and SD; lines in FTV and TLF groups represent median and interquartile range. P < 0.05 indicates significant difference. ns = not statistically significant.
DISCUSSION
In the present study, we aimed to assess the nodal stage performance of 68Ga-FAPI-04 PET/CT in NSCLC and to determine whether 68Ga-FAPI-04 tumor uptake could accurately reflect FAP expression. Our results indicated that 68Ga-FAPI-04 had excellent performance for identifying mediastinal and hilar lymph node metastases, and volume-based PET parameters showed a significant correlation with FAP expression in primary NSCLC tumors.
It is important to make an accurate diagnosis of pulmonary lesions, to inform subsequent treatment options and clinical prognosis. 68Ga-FAPI-04 recently demonstrated high accuracy for the detection of primary tumors in lung cancer, ranging from 90% to 100% (11,12,14). Moreover, 68Ga-FAPI uptake may be related to the pathologic type of tumor, and the current study accordingly found that SUVmax, FTV, and TLF were all higher in SCCs and other rare tumor types than in adenocarcinomas. Both 68Ga-FAPI and 18F-FDG revealed similar detection efficiencies for most solid lesions. Nonsolid nodules were a major cause of false-negative findings, with a detection rate of 75% (6/8) in our study; however, compared with 18F-FDG, 68Ga-FAPI-04 had better detection efficiency (77.8% [21/27] vs. 40.7% [11/27]) and a higher SUVmax (4.1 vs. 2.8) for detecting nonsolid nodules (<3 cm) (12). 68Ga-FAPI-04 may thus be a good alternative tracer for the diagnosis of NSCLC.
Accurate evaluation of lymph node involvement is crucial in choosing the treatment strategy for patients with early-stage or resectable NSCLC. 18F-FDG PET/CT has significantly improved the accuracy of lymph node staging of NSCLC compared with contrast-enhanced CT; however, false-positive (inflammatory diseases of lymph nodes) and false-negative (microscopic lymph node metastases) findings limit the diagnostic effectiveness of 18F-FDG for the N-stage of NSCLC, leading to relatively low sensitivity (49.6%–72.2%) and specificity (81.5%–95%) in per-station analysis (22,23). Compared with 18F-FDG, 68Ga-FAPI showed better diagnostic efficiency for detecting lymph node metastasis, especially in terms of diagnostic specificity (97.6%–99% vs. 6%–88.8%) in NSCLC, whereas metastatic lymph nodes also had a higher SUVmax than nonmetastatic ones for 68Ga-FAPI (10.3 vs. 2.9), but not 18F-FDG (12,14). Similar to previous studies, we also demonstrated a high specificity of 93.10% indicating the value of 68Ga-FAPI-04 for diagnosing true-negative lymph nodes. The false-negative lymph nodes detected by 68Ga-FAPI-04 are mainly due to small nodules (short diameter < 0.5 cm), necrosis, and micrometastases. The present study identified some false-positive nodes, and the pathologic results showed many accumulated macrophages and phagocytosed carbon particles in the lymph node, suggesting that it may be related to FAP expression in macrophages and the individual’s working environment. Our results showed that 68Ga-FAPI-04 had an accuracy of 67.74% (21/31) for N-staging in surgical NSCLC, which was slightly lower than in a previous study (80% [8/10]) (14). Overall, these findings suggest that 68Ga-FAPI-04 has good potential for detecting lymph node involvement, thus guiding the surgical strategy for lymph node dissection in patients with early-stage or resectable NSCLC.
FAP is closely associated with tumor progression and a worse clinical outcome (24,25). FAP expression levels are important in terms of FAP targeting PET imaging and radionuclide therapy in patients with lung cancer. The reported FAP-positivity rate in NSCLC is 76.2%–97.3%, with variations in expression levels among histopathologic types (24,26). Wei et al. demonstrated a moderate correlation between 18F-FAPI–derived SUVmax and FAP expression in 6 surgical and 26 biopsy lung cancer specimens (r = 0.439) (15). However, our results confirmed that volume-based PET parameters (FTV and TLF) were strongly associated with FAP expression, suggesting that tumor uptake burden can reflect FAP expression more accurately than SUV in NSCLC. We also found that most SCCs (12/15) showed intense FAP expression, higher 68Ga-FAPI-04 uptake, and a sharp outline, partially consistent with the results of Moreno-Ruiz et al. (25). FAP expression in adenocarcinomas varied greatly, with weak expression in highly differentiated adenocarcinomas. Additionally, adenosquamous and sarcomatoid carcinomas showed strong FAP expression, whereas FAP expression was weak in most large cell neuroendocrine carcinomas and small cell lung cancers. The strong FAP expression and high 68Ga-FAPI-04 uptake (SUVmax, 12.85 ± 6.69) in benign lung disease (e.g., organizing pneumonia, tuberculosis, and cryptococcosis) revealed by our study suggest that we should pay more attention to these in routine clinical practice. Overall, 68Ga-FAPI–derived FTV and TLF may thus accurately reflect the expression level of FAP, which could serve as a predictive indicator for FAP-targeted radionuclide therapy in patients with advanced NSCLC.
Our study had some limitations. First, the diagnosis of some distant metastatic lesions was based mainly on the typical imaging performance and lacked supporting histopathologic evidence. Second, the subgroup analysis sample (SCC) for FAP immunostaining was relatively small, which may affect the accuracy of the results. Third, the sample size for FAP and GLUT-1 double staining was also small, and the data may not be representative. Further large-scale multicenter prospective clinical trials are therefore required to validate the current findings regarding 68Ga-FAPI-04 in NSCLC.
CONCLUSION
68Ga-FAPI-04 PET/CT can accurately identify lymph node involvement, which is important for informing the choice of surgery in participants with resectable NSCLC. Tumor 68Ga-FAPI-04 uptake correlated with FAP expression in NSCLC, especially in terms of the volume parameters (FTV and TLF). 68Ga-FAPI-04 may become a valuable alternative tracer agent for imaging NSCLC, and 68Ga-FAPI-derived volume parameters may be potential predictors of FAP-targeted radioligand therapy in patients with advanced NSCLC.
DISCLOSURE
This study received funding from the Improvement Project for Theranostic Ability on Difficulty Miscellaneous Disease (Tumor) (ZLYNXM202007), the Medical Sci-Tech Innovation Platform of Zhongnan Hospital of Wuhan University (PTXM2022013), the National Natural Science Foundation of China (82372005 and 82171986), Hubei Provincial Natural Science Foundation (2022CFB169), and Hubei Province Health and Family Planning Scientific Research Project (WJ2023M051). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does 68Ga-FAPI-04 PET accumulation correlate with FAP expression in NSCLC?
PERTINENT FINDINGS: 68Ga-FAPI-04 PET/CT revealed excellent diagnostic efficiency, and 68Ga-FAPI–derived volumetric parameters showed strong correlations with FAP expression in NSCLC.
IMPLICATIONS FOR PATIENT CARE: Our findings may accelerate the clinical translation of FAP-targeted radioligand therapy in patients with advanced NSCLC.
Footnotes
↵* Contributed equally to this work.
Published online Mar. 7, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 5, 2023.
- Revision received February 13, 2024.