Abstract
T14
Introduction: The objective is to evaluate three infusion methods - syringe pump, gravity and peristaltic pump - for safety, ease of identification of the start and end of the therapeutic radiopharmaceutical (TRP) infusion, while adhering to the ALARA principle.
Here we report on our experience infusing investigational and FDA-approved TRPs, by evaluating intravenous administrations from a syringe, single vial, and multiple vials, using the aforementioned infusion methods.
Methods: Syringe pump (MedFusion 4000 and Graseby 3100): The size of the syringe containing the TRP for infusion is identified by the pump. A three way stop cock is employed to link the syringe containing the TRP, a sterile saline flush and extension tubing leading to the patient's intravenous catheter. The pump can be programed to infuse the dose based on syringe size, volume of the TRP and duration of administration.
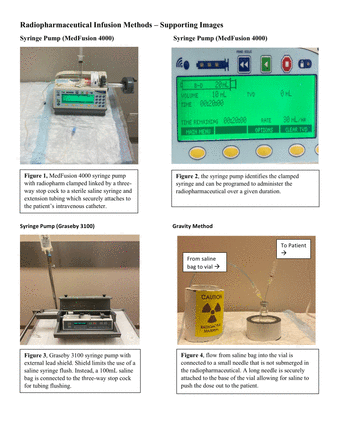
Gravity method: A sterile saline bag with extension tubing connected to a short needle which is inserted into the vial containing the TRP is used, while ensuring the short needle is not submerged. Then, a long needle securely attached to the base of the vial allows for saline to push the dose out to the patient. The longer needle is connected to extension tubing leading to the patient's intravenous catheter. The saline drip rate determines the duration of the infusion.
Peristaltic pump (Carefusion Alaris PC): A sterile saline bag connected to the pump tubing which is secured to the patient's intravenous catheter is used. A long needle coupled to extension tubing links the TRP vial to the pump tubing for infusion. The peristaltic pump can be programmed to administer the dose from a single vial or multiple vials over a given duration.
Results: The syringe pump is designed to identify the syringe attached to it and mechanically push a predetermined dose volume at a specific rate. Once the pump has completed this programmed infusion, the syringe is empty, but radiopharmaceutical remains in the extension tubing. This indicates that the infusion is not continuous, requires staff to manually flush the tubing with saline to infuse the total dose volume, and leads to additional exposure during the saline flush. If a syringe shield is used, the syringe pump misregisters the attached syringe size, which could lead to an unintended change in the duration of administration. The Graseby pump has an external lead shield that encompasses the entire pump but is heavy and cumbersome to move weighing approximately 90 lbs.
The gravity method is a continuous infusion from a vial, whereby staff can observe the infusion from a distance, but it is difficult to accurately determine the start and end of infusion, because the gravity method dilutes the TRP with saline over a given duration.
The peristaltic pump allows for a continuous administration from a vial whereby staff can observe the infusion from a distance. Staff can program the peristaltic pump for a specific duration based on the total volume to be infused which consists of the radiopharmaceutical volume and the volume of the infusion tubing. Staff can accurately identify the start and end of infusion based on the dosage volume to be administered. Multi-vial application, up to three vials, can be sequentially administered providing staff the ability to safely administer high volume TRPs safely and continuously providing high quality data.
Conclusions: The peristaltic pump is optimal for administering therapeutic radiopharmaceuticals continuously and can be employed for single or multiple vials while being able to accurately identify the start and end of infusion.