Abstract
P1300
Introduction: This is a Phase I, first-in-human study to evaluate the safety of the radiotheranostic peptide pair, [68Ga]Ga DOTA-5G and [177Lu]Lu DOTA-ABM-5G in patients with locally advanced or metastatic pancreatic adenocarcinoma (PDAC). [68Ga]Ga DOTA-5G is an αvβ6-integrin-targeted peptide, radiolabeled with gallium-68, that is being developed as a diagnostic imaging agent. [177Lu]Lu DOTA-ABM-5G contains the same αvβ6-integrin-targeted peptide appended with an albumin binding moiety (ABM) to extend the circulation half-life, is radiolabeled with lutetium-177, and is being developed as a radiotherapeutic. PET/CT imaging with [68Ga]Ga DOTA-5Gis being used diagnostically to select patients that have αvβ6-avid disease who are then be eligible for treatment with the [177Lu]Lu DOTA-ABM-5G. SPECT/CT imaging is being used to quantify the whole body dosimetry for the [177Lu]Lu DOTA-ABM-5G. The hypotheses of this Phase I study are that a)[68Ga]Ga DOTA-5G detects lesions in patients with locally advanced or metastatic PDAC b) the theranostic pair [68Ga]Ga DOTA-5G/ [177Lu]Lu DOTA-ABM-5Gis safe and well tolerated and c) we are able to identify a Recommended Phase 2 Dose (RP2D) for [177Lu]Lu DOTA-ABM-5Gtherapy to be used in subsequent Phase II trials.
Methods: Patients with metastatic or locally advanced PDAC who demonstrated advanced disease refractory to at least one prior standard-of-care treatment were recruited to the study. Patients received an intravenous injection (IV) of up to 5 mCi of [68Ga]Ga DOTA-5G; PET/CT images were acquired 1 hour post injection (p.i.) and blood samples were obtained at serial time points throughout the uptake period (at approximately 5, 10, 15, 30, 60 min p.i.). Vital signs, blood samples and EKG were checked 1 and 7 days after injection of [68Ga]Ga DOTA-5G.. Patients with lesions with an SUVmax ([68Ga]Ga DOTA-5G ) >2-fold above normal lung, or liver were eligible for [177Lu]Lu DOTA-ABM-5G treatment. Eligible patients received a single treatment with [177Lu]Lu DOTA-ABM-5G (25-150 mCi, 3+3 dose escalation) and underwent whole body planar imaging (anterior and posterior view) and SPECT/CT imaging at approximately 1 and 7-days following administration of [177Lu]Lu DOTA-ABM-5G, for evaluation of biodistribution and dosimetry. Organs-at-risk (kidneys, bone marrow) were contoured on CT to extract the Lu-177 activity and organ volume, which were used in OLINDA/EXM 1.1 to calculate the absorbed dose. Vital signs, blood samples and EKG were checked 1, 7 and 14 days after [177Lu]Lu DOTA-ABM-5G treatment.
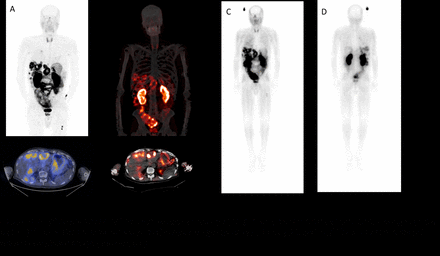
Results: To date, 17 patients have been imaged with [68Ga]Ga DOTA-5G and 14 patients have been treated with [177Lu]Lu DOTA-ABM-5G. Both the [68Ga]Ga DOTA-5Gand [177Lu]Lu DOTA-ABM-5G agents were well tolerated and no drug related serious adverse events have been observed. The [68Ga]Ga DOTA-5G was predominantly renally excreted and [68Ga]Ga DOTA-5G PET/CT imaging was able to detect bone, lung, and hepatic metastasis. The [177Lu]Lu DOTA-ABM-5G was also predominantly renally excreted and taken up and retained by the lesions detected by [68Ga]Ga DOTA-5G PET/CT imaging (representative images shown in Figure 1, ). The average absorbed dose to kidneys and bone marrow (respectively) obtained to date are 3.7 and 0.01 Gy for dose level 1 (25mCi, 26.3 mCi average ), 6.93 and 0.02 Gy for dose level 2 (50 mCi, 52.7 mCi average), 10.03 and 0.03 Gy for dose level 3 (100 mCi, 99.5 mCi average) and 14.56 and 0.04 Gy for dose level 4 (150 mCi, 141 mCi average) estimated using OLINDA/EXM 1.1 (n=3 patients/ dose level). Currently patients are being enrolled for treatment at the maximum dose level of 200 mCi.
Conclusions: To date we have demonstrated that [68Ga]Ga DOTA-5G PET/CT can be used to visualize disease and select patients for treatment, that the [177Lu]Lu DOTA-ABM-5G reaches and is retained by the metastasis and that the theranostic pair [68Ga]Ga DOTA-5G and [177Lu]Lu DOTA-ABM-5Gis safe and well tolerated.