Abstract
The aim of the study was to use dual-isotope lymphoscintigraphy in healthy volunteers and women with breast cancer–related lymphedema (BCRL) to detect and quantify transport of radiolabeled protein from a subcutaneous injection depot to local blood vessels as a potential mechanism of protection against edema resulting from treatment to the axilla. Methods: A total of 29 subjects and 18 women with a history of BCRL received bilateral subcutaneous injections of human IgG (HIgG) in the second dorsal web space of each hand, 99mTc-HIgG on one side and 111In-HIgG on the other. In 8 further healthy subjects, epinephrine was administered with the labeled HIgG. Radioactivity at each depot was measured at regular intervals for a total of 3 h using a collimated sodium iodide scintillation detector, and radioactivity in venous blood sampled from both arms was measured using an automatic sample counter. Ipsilateral blood time–concentration curves were corrected for recirculating activity by subtraction of the simultaneous contralateral concentration, to define the component of ipsilateral blood resulting from local vascular access of radioprotein. Accumulation of activity in blood was expressed in relation to injected activity and activity that had left the depot and was calculated as a function of time—in systemic blood, by multiplying contralateral concentrations by an estimate of the subject’s blood volume, and in ipsilateral blood, by using indicator dilution theory and an assumed forearm blood flow of 20 mL/min. Results: 99mTc-HIgG and 111In-HIgG behave almost identically with respect to depot clearance and accumulation in contralateral venous blood, with or without epinephrine, which reduced both depot clearance and blood accumulation rate. Moreover, a side-to-side correlation with respect to contralateral accumulation was present in healthy subjects, was not abolished by epinephrine, and was maintained in the face of asymmetric accumulation in BCRL. Contralateral accumulation of radioprotein was reduced in BCRL after injection into the affected side only when the hand was involved. In contrast to contralateral sampling, ipsilateral time–concentration and accumulation profiles were consistent with instability of 111In-HIgG and rapid local vascular access of small amounts of protein-free 111In. Experiments based on precipitation of protein with trichloroacetic acid confirmed relatively high levels of unbound ipsilateral 111In, especially in samples obtained early after injection. Substantial accumulation of protein-bound 99mTc was observed in ipsilateral blood, with a time course similar to that of contralateral accumulation. Positive correlation between ipsilateral and contralateral blood 99mTc activity was observed at all time points, often significantly, in contrast to 111In, for which it was negative at all time points. Ipsilateral accumulation of 99mTc adjusted for activity that had left the depot was unchanged with respect to the affected arm in BCRL patients. Conclusion: Whereas 111In-HIgG and 99mTc-HigG are interchangeable for measurement of depot clearance and contralateral venous accumulation rates, ipsilateral sampling is much more sensitive to protein-free radionuclide and detects significant differences resulting from some instability of 111In-HIgG. On the basis of 99mTc data, there appears to be substantial local vascular access of radioprotein within the arm, both in healthy subjects and in patients with BCRL, through either lymphaticovenous communications or direct transendothelial transport.
Breast cancer–related lymphedema (BCRL) remains a common complication of breast cancer treatment, occurring in approximately 25% of cases (1). Although it is clear that the primary initiating event is surgery or radiotherapy to the axilla, the actual pathophysiologic changes involved are poorly understood (2). They would have to account for several apparently anomalous observations, including the fact that BCRL does not develop in most women treated for breast cancer and the phenomenon of “sparing,” in which parts of an otherwise swollen arm (often the hand) remain unaffected.
An attractive explanation for these conundrums would be a variable degree of protein entry to local blood vessels within the affected arm, either through anatomic lymphaticovascular communications or through direct crossing of blood capillary endothelium, providing an alternative route for protein transport into blood in the event of obstruction at the axilla. Several animal studies have raised a strong suspicion of the existence of lymphaticovascular communications (3–8). Moreover, Aboul-Enein et al. (9), in a clinical study, suggested that anatomic lymphaticovenous communications in the arm might protect women from BCRL by providing an alternative transport route for interstitial protein.
Although protein transport between the interstitial space and blood is generally thought to be essentially unidirectional from tissue to blood, transport in the opposite direction could provide another mechanism for protein to return to blood without having to transit the axilla, and a few studies support physiologic transport in this direction (10–12).
Cannulation of lymphatic vessels is technically demanding and invasive and risks further exacerbation of lymphatic impairment (13). We previously described a new technique of dual-isotope lymphoscintigraphy demonstrating virtually identical behavior between 111In-labeled human polyclonal human IgG (HIgG) and 99mTc-HIgG for clearance rate from a subcutaneous injection depot and accumulation rate in mixed venous blood (14,15). The aim of this study was to apply this technique to determine the extent to which radioprotein injected interstitially can access local blood vessels, both in healthy volunteers and in women with BCRL. By sampling blood from both ipsilateral and contralateral veins, we could correct ipsilateral time–concentration curves for recirculating activity and therefore accurately define the component of the ipsilateral curve that is exclusively the result of local entry of radioactivity. Because of concern that local access to blood may be influenced by needle trauma to blood vessels at the site of depot injection, a subset of healthy volunteers in whom epinephrine was coinjected with radioprotein was studied with the aim of constricting damaged blood vessels and minimizing any artifactual access.
MATERIALS AND METHODS
Subjects
Three groups of subjects were studied: 18 patients with unilateral BCRL, 29 control subjects (of whom 23 had breast cancer but had not had surgery and 6 were healthy volunteers) and 8 healthy volunteers in whom epinephrine was coinjected with radiolabeled HIgG. Because of the possibility of nodal involvement and any resulting effect on lymphatic function in the arm, only arms opposite the side of the breast cancer were included from the 23 (preoperative) volunteers with breast cancer. All subjects gave informed consent to the procedure, which was approved by the Local Research Ethics Committee.
Radioprotein Preparation
99mTc-HIgG was prepared by the addition of 99mTc-pertechnetate to kits that contained 1 mg of 2-iminothiolane–derivatized human IgG and 8 mg of stannous chloride (Technescan HIG; Mallinckrodt Medical BV), as previously described (14,15). For labeling with 111In, human immunoglobulin (Sandoglobulin; Novartis Pharmaceuticals U.K. Ltd.) was derivatized with diethylenetriaminepentaacetic acid cyclic anhydride as described by Hnatowich et al. (16). Before injection, 99mTc-HIgG and 111In-HIgG were diluted to 10 MBq/mL and 5 MBq/mL, respectively, with 0.1 mol of sodium bicarbonate per liter containing 5 mg of HIgG per milliliter, and 0.2-mL volumes were drawn into tuberculin syringes with 25-gauge needles.
In 8 healthy volunteers, the 0.2-mL dose contained 0.02 mg of epinephrine. In vitro studies demonstrated that whereas the stability of 99mTc-HIgG was not affected over 4 h, epinephrine did reduce the radiochemical purity of 111In-HIgG by 7% over 30 min, which was the maximum delay between preparation and administration of the mixture.
Depot Clearance
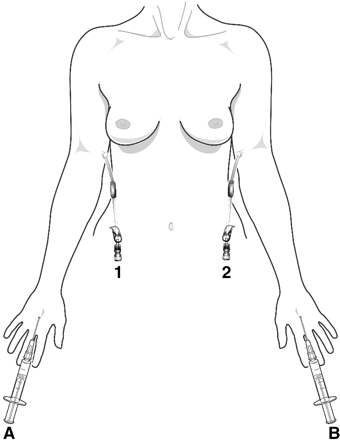
99mTc-HIgG was injected in the second dorsal web space of one hand and 111In-HIgG in a similar site of the opposite hand, each administered subcutaneously in a volume of 0.2 mL via a 25-gauge needle (Fig. 1). Clearance rate from the site of depot injection was measured with a collimated sodium iodide scintillation detector.
Experimental setup for venous sampling. Subcutaneous injections of radiolabeled HIgG were made bilaterally (A and B), with 111In-HIgG injected through A and 99mTc-HIgG through B, followed by bilateral venous sampling. Blood sampled from catheter 1 gave the ipsilateral 111In time–concentration curve and the contralateral 99mTc curve, and blood sampled from catheter 2 gave the ipsilateral 99mTc time–concentration curve and the contralateral 111In curve. Both contralateral curves were subtracted from the corresponding ipsilateral curves to correct the ipsilateral curves for recirculation. In some patients, A was used for 111In-HIgG and B for 99mTc-HIgG, and vice versa in others.
Venous Sampling
Twenty-gauge venous cannulae were sited in the medial cubital vein at the antecubital fossa bilaterally (Fig. 1). Venous samples were taken 15 min after depot injection and at frequent intervals up to 3 h. All samples were prepared for counting in an automatic γ-counter (1282 CompuGamma; LKB-Wallac) as previously described (14,15).
Postinjection Analysis of Radionuclide Binding to Plasma Protein
In a subgroup of 5 subjects (all controls), the proportion of radioactivity in blood that was bound to protein was determined by a modification of the protein-bound iodine test (17). A total of 1.5 mL of 5% trichloroacetic acid was added to 1-mL aliquots of plasma. The tubes were mixed and then centrifuged at 1,000g for 5 min. After the supernatant was decanted, the pellet was washed with 2.5 mL of 5% trichloroacetic acid and dissolved in 2.5 mL of 2N sodium hydroxide. Radioactivity in the supernatant fractions and washings and in the final pellet was measured in the automatic γ-counter, and percentage activity in the pellet was calculated.
Data Analysis
Blood Accumulation Rate.
Blood concentrations of radiolabeled HIgG, sampled from the contralateral antecubital vein, were recorded as percentage of administered activity per liter of blood. The total amount of circulating radioactivity, expressed as a percentage of administered activity, was obtained by multiplication of the blood concentration in each sample by the subject’s blood volume, in liters, estimated from height, weight, sex, and age using a standard conversion equation (18). The total blood accumulation rate, as determined from contralateral sampling, was essentially linear and therefore fitted by linear regression analysis to give a slope, bcontra, having units of percentage of administered dose per minute. This blood accumulation rate will depend on the amount of radioprotein that has left the depot and is available for transport. Thus, to focus on the efficacy with which protein is transported from the interstitial space to blood, we divided bcontra by the fraction of activity that had left the depot over the 3-h observation period to give the depot-corrected value corrbcontra.
Blood concentrations of radiolabeled HIgG, sampled from the ipsilateral antecubital vein, were also recorded as percentage of administered activity per liter of blood. The contralateral time–concentration curve was subtracted from the ipsilateral curve to record a curve that is corrected for recirculating activity. Using the principle of indicator dilution (i.e., principle of conservation of mass as in the Stewart–Hamilton equation for measurement of blood flow), the recirculation-corrected ipsilateral time–concentration curve was then integrated over 3 h and compared with an assumed value for local forearm blood flow, contributing to the dilution of radioprotein, to estimate the total amount of radioactivity, M, accumulating in local ipsilateral blood as a function of time; that is, M(t) = blood flow × area under the ipsilateral time–concentration curve(t), where t is time. Relevant forearm blood flow was assumed to be 20 mL/min, based on a forearm volume estimate of 1 L and a forearm blood perfusion of 2 mL/min/100 g (19). As for contralateral sampling, the profile of radioprotein accumulating ipsilaterally was fitted by linear regression analysis to give bipsi, which is the rate of ipsilateral protein accumulation in units of percentage of administered activity per minute. Like bcontra, bipsi was corrected for the amount of radioprotein that had left the injection depot over 3 h, to give corrbipsi.
Depot Clearance Rate.
The count rates recorded at each time point at the depot of radiolabeled HIgG were fitted with a single exponential, and the rate constant, k, was calculated. The value of k depends on several processes, including diffusion in the interstitial fluid away from the depot and possible direct access to local blood capillaries, but is generally assumed to depend largely on local lymph flow, quantification of which is routinely based on k in clinical lymphoscintigraphy (20,21).
Statistics
Parametric statistics were used. Values are expressed as mean, followed by SEM in parentheses. The Student paired or unpaired t test was used to compare mean values, and the Pearson correlation coefficient was used to evaluate associations between parameters. A P value of less than 0.05 was regarded as significant.
RESULTS
Control Subjects and Epinephrine Recipients
In the control arms, there was no significant difference between k based on 99mTc-HIgG (n = 12) and k based on 111In-HIgG (n = 22), with respective mean values of 0.13 (0.01) and 0.14 (0.01) 102 min−1. Depot clearance was reduced in the presence of coinjected epinephrine and was slightly but significantly lower with 99mTc-HIgG than with 111In-HIgG, with respective mean values of k of 0.074 (0.009) and 0.094 (0.012) 102 min−1 (P < 0.05). As with k in control arms, 99mTc-HIgG and 111In-HIgG did not significantly differ in total blood accumulation rate (bcontra) (Fig. 2), with respective mean values of 0.065% min−1 (0.006% min−1) and 0.062% min−1 (0.004% min−1). Although reduced in the presence of coinjected epinephrine, bcontra did not differ between 99mTc-HIgG and 111In-HIgG, with respective mean values of 0.038 (0.004) and 0.041 (0.004) 102 min−1.
Accumulations of 99mTc and 111In (expressed as percentage of injected activity) in ipsilateral (corrected for recirculation) and contralateral venous blood as a function of time after injection, with and without coadministration of epinephrine. For clarity, error bars (SEM) are shown only for 3-h values (Table 1 provides more detail on variance). Note the different vertical scales for ipsilateral and contralateral profiles.
In control subjects and subjects receiving coinjected epinephrine, 111In-HIgG and 99mTc-HIgG clearly correlated with respect to contralateral radioprotein accumulation at all individual time points after 15 min (Fig. 3). The coefficients of these correlations, which reached significance after 30 min both with and without epinephrine (Fig. 3), increased over time.
Contralateral radioprotein concentrations display a side-to-side correlation (i.e., 99mTc-HIgG vs. 111In-HIgG) at all but the earliest time points. This figure shows the strength of that correlation as a function of time after injection for controls without coadministration of epinephrine, controls plus epinephrine, and patients with BCRL. The r values shown alongside each subgroup in the key refer to the relationship between time after injection and the correlation coefficients recorded at individual time points. The n values in the key refer to the numbers of individuals on which the correlation coefficients are based. In general, the strength of side-to-side correlation increased over time for all 3 groups of subjects.
Radioactivity accumulating in ipsilateral blood over 3 h was considerable and, in the case of 99mTc-HIgG, amounted to 4.5% (0.56%) of the administered activity at 3 h in control arms, compared with 10.1% (0.9%) in central blood (sampled contralaterally) (Fig. 2). In the case of 111In-HIgG, ipsilateral accumulation at 3 h was 3.0% (0.33%) of administered activity (P < 0.05 vs. ipsilateral 99mTc-HIgG), compared with 10.0% (0.8%) in central blood (Fig. 2). Simultaneous administration of epinephrine reduced ipsilateral accumulation at 3 h to 2.3% (0.4%) of the administered activity for 99mTc and to 1.8% (0.35%) for 111In (Fig. 2), compared with accumulations in central blood of 6.01% (0.56%) and 6.9% (0.71%), respectively, at 3 h after coinjected epinephrine.
In contrast to depot clearance and contralateral blood accumulation rates, the time courses of (nonintegrated) ipsilateral blood concentration clearly differed between the 2 radiopharmaceuticals (Fig. 4). Thus, the concentration of 111In decreased nonlinearly over time with or without epinephrine, whereas the concentration of 99mTc increased over time. These differences are reflected in the cumulative (i.e., integrated) profiles, which, in the case of 99mTc, displayed time courses similar to the corresponding contralateral cumulative profiles but in the case of 111In were convex upward, with time courses clearly different from the corresponding contralateral profiles (Fig. 2).
Time course of venous radioprotein concentration in blood sampled ipsilaterally and corrected for recirculation. The effects of coadministration of epinephrine are shown.
These differences in kinetics suggest artifactual behavior, most likely instability of binding. This suspicion was confirmed by measurement of the fraction of radioactivity that was protein bound in ipsilateral and contralateral blood. Thus, as Figure 5 shows, whereas in contralateral blood virtually all the radioactivity was protein bound throughout, significant quantities of protein-free 111In were present in ipsilateral blood, especially early after injection. Consistent with the presence of free 111In, there was a consistent inverse correlation, which became stronger over time, for each time point between ipsilateral and contralateral cumulative 111In levels in control subjects (Fig. 6). In contrast, the corresponding correlations for 99mTc were positive, albeit weakening over time. In other words, the higher the ipsilateral accumulation of 111In, the lower the contralateral accumulation of 111In.
Comparison between concentrations of total radioactivity and protein-bound radioactivity in ipsilateral blood (corrected for recirculation) (A) and contralateral blood (B) after administration of 99mTc-HIgG and 111In-HIgG in 5 control subjects. Error bars are ±SEM.
Ipsilateral protein concentration (corrected for recirculation) and contralateral concentration at individual time points after injection correlated in a way that was clearly different for 99mTc-HIgG than for 111In-HIgG (the number of paired observations on which each point is based was 21 or 22 for 99mTc and 22 or 23 for 111In). Thus, for 99mTc-HIgG the correlation between ipsilateral and contralateral concentrations was always positive, whereas for 111In-HIgG it was always negative. Moreover, for 99mTc-HIgG the strength of the (positive) correlation decreased significantly over time, whereas for 111In-HIgG the strength of the (negative) correlation increased significantly over time. As in Figure 2, the r values in the key refer to the strength of the relationship between time after injection and the correlation coefficients at individual time points. These findings are consistent with the presence of free 111In early after injection and with free 99mTc emerging late after injection. *P < 0.05.
In view of the early high levels of free 111In in ipsilateral blood, the lower ultimate accumulation of 111In in ipsilateral blood, and the inverse correlation between 111In accumulating in, respectively, ipsilateral and contralateral blood, subsequent analysis of data on ipsilateral sampling was restricted to studies based on 99mTc-HIgG. Accordingly, in control subjects, bipsi based on 99mTc-HIgG was 0.03% min−1 (0.004% min−1) and, in recipients of epinephrine, was 0.012% min−1 (0.002% min−1) (P < 0.01). After correction for depot clearance, corrbipsi based on 99mTc-HIgG was 0.138% min−1 (0.021% min−1). In recipients of epinephrine, it was 0.11% min−1 (0.031% min−1) (n = 7, excluding an outlying subject in whom corrbipsi was inexplicably high, at 0.92% min−1), which was not significantly different from controls.
Patients with BCRL
Although bcontra was higher for the unaffected (nonswollen) arm than for the affected arm, side-to-side correlation was maintained despite previous axillary surgery and subsequent development of edema (Fig. 3). This correlation became strongly significant after 30 min.
Values for bcontra in BCRL are shown in Table 1, which also shows values for corrbcontra in all BCRL patients, including subgroup analysis with respect to involvement or sparing of the hand. Mean corrbcontra (based on pooled 111In and 99mTc data) was less in affected arms (for swollen and spared hands combined) than in control arms (P < 0.01) or the contralateral arm (P < 0.05) (Table 1). On subgroup analysis, corrbcontra in arms in which the hand was spared was similar to that in controls, but when the hand was involved, corrbcontra was significantly less than in controls (P < 0.001).
Mean (SEM) Values (% min−1) for bcontra, bipsi, corrbcontra, and corrbipsi
Mean bipsi was significantly less in affected arms than in contralateral unaffected arms (P < 0.05), but corrbipsi in affected arms was not significantly different from either contralateral unaffected arms or arms of control subjects (Table 1). Data were insufficient for subgroup analysis.
DISCUSSION
Contralateral (i.e., systemic blood) recovery after subcutaneous injection of radiolabeled HIgG in healthy subjects and patients with BCRL has been reported elsewhere (14,15,22). The purpose of the current study was to examine the efficacy with which radioprotein that has left the injection depot accesses the vasculature, both locally (as determined from ipsilateral sampling) and by whatever route (as determined from contralateral sampling).
Although 99mTc-HIgG and 111In-HIgG have similar depot clearance rates and time–activity profiles in contralateral blood samples, different profiles between the radiopharmaceuticals have been found in ipsilateral blood (Figs. 2 and 4). Moreover, whereas the time course of total 99mTc accumulation in ipsilateral blood was similar to that in systemic blood, the time course of 111In accumulation in ipsilateral blood differed from that in contralateral blood. Relatively more 111In than 99mTc accumulated in ipsilateral blood early after injection, suggesting release and rapid vascular access of a small radiolabeled complex, presumably 111In-diethylenetriaminepentaacetic acid. This suggestion is supported by the finding of more protein-free 111In in ipsilateral blood in early samples (Fig. 5) and lower ultimate levels of accumulation of 111In ipsilaterally (Fig. 2).
The correlation of cumulative levels of the same radionuclide between ipsilateral and contralateral blood at each time point after injection was positive for 99mTc (as would be expected if some radioprotein reached the systemic circulation via local vascular uptake as well as via the major lymphaticovenous communications in the neck) but negative for 111In (Fig. 6). Although the correlation for 99mTc weakened with time, that for 111In (while remaining negative) strengthened. In the absence of protein-free tracer, a positive correlation would be expected if protein escaped from the arm in ipsilateral blood, with a coefficient that should increase with time as a result of improving data statistics, as was observed when the 2 contralateral cumulative activities were compared (Fig. 3). The strength of ipsilateral versus contralateral correlation should depend on the relative contribution of local access to systemic blood levels and the variance of local access from subject to subject, with no correlation if local access is negligible. Because of the greater diffusibility and easier vascular access of small molecules, free tracer would increase ipsilateral activity. On the other hand, faster clearance of small molecules from systemic blood would tend to lower contralateral activity and therefore promote a negative correlation between ipsilateral and contralateral blood levels, as was seen with 111In-HIgG. The conclusion from this study, therefore, is that 111In-HIgG is less stable than 99mTc-HIgG, at least early on. Although there is evidence of later minimal dissociation of 99mTc (as seen, for example, in the decreasing correlation between ipsilateral and contralateral radioprotein concentrations [Fig. 6]), this dissociation does not appear to invalidate 99mTc-HIgG (at least not completely) for ipsilateral sampling (Fig. 5), nor does it appear to invalidate either radioprotein for contralateral sampling. One can predict that ipsilateral sampling would be highly sensitive to protein-free tracer if all protein-free activity were crystalloid and entered exclusively blood vessels, rather than lymphatics. Thus, if we started with 97.5% of injected activity protein bound and 10% of this activity were recovered in central blood over 3 h (speculatively, 7.5% by central lymphaticovenous communications and 2.5% by local vascular access), then on average, over 3 h, only 50% of activity in ipsilateral blood would be protein bound.
The molecular size of HIgG (molecular weight, 150 kDa; Stokes–Einstein radius, 5.5 nm) makes it too large to pass through capillary endothelial pores in significant quantities by passive diffusion. Experimental evidence nevertheless suggests that significant quantities of plasma protein (11) and IgG (12) cross the endothelium from the interstitial space to intraluminal blood instead of entering lymphatic vessels, possibly by vesicular transport (10). Anatomic lymphovenous communications (6–9) may provide another mechanism.
From the current study, evidence (restricted to 99mTc data) favoring local vascular access, by whatever mechanism, is as follows.
First, a considerable amount of radiotracer, mostly protein bound, accumulated in ipsilateral blood, and this accumulation followed an almost identical time course to that of activity in the central circulation. This finding, on the face of it, would appear to account for approximately 30% of activity appearing in mixed venous blood in control arms (Fig. 2), if our assumption that forearm blood flow contributed to radiotracer dilution is accurate. This estimate, however, would need to be adjusted in proportion to estimates of protein binding in ipsilateral blood, and a more realistic estimate may be about 15%.
Second, a positive correlation between ipsilateral and contralateral blood was observed at all time points (Fig. 6) and was significant at early time points, implying that ipsilateral accumulation influences contralateral accumulation.
Third, even at 15 min, a side-to-side correlation emerged for activity in contralateral venous samples and was not abolished by breast cancer treatment and the development of BCRL (Fig. 3).
Finally, in BCRL arms in which the hand was swollen, the rate of accumulation of activity in contralateral venous samples, corrbcontra, was significantly less than in controls. This finding is consistent with the traditional “stopcock” theory for the etiology of BCRL. However, when the hand was spared, corrbcontra did not lessen significantly, despite disruption at the axilla sufficient to produce swelling in the rest of the arm. Protein injected in the dorsal web space of these patients either had access to lymphatic pathways not available to the rest of the arm or else entered blood directly within the arm. This study, however, did not show that development of BCRL increased such access.
CONCLUSION
We conclude that 99mTc-HIgG and 111In-HIgG are of unequal labeling stability. With respect to rates of accumulation after subcutaneous injection, the 2 radioproteins remain effectively interchangeable for systemic venous blood but not for ipsilateral blood, as a result of amplification of the small amounts of free, crystalloid label that gain local vascular access. However, our findings suggest that BCRL has a pathophysiology more complex than simple obstruction to lymphatic flow in the axilla. Local protein access to blood within the arm needs to be taken into account.
Acknowledgments
The support of the Wellcome Trust is gratefully acknowledged.
Footnotes
Received Aug. 7, 2003; revision accepted Dec. 29, 2003.
For correspondence or reprints contact: A. Michael Peters, MD, Department of Nuclear Medicine, Box 170, Addenbrooke’s Hospital, Hills Rd., Cambridge CB2 2QQ, U.K.
E-mail: michael.peters{at}addenbrookes.nhs.uk.