Visual Abstract
Abstract
Understanding the relationship between lesion-absorbed dose and tumor response in 177Lu-PSMA-617 radiopharmaceutical therapies (RPTs) remains complex. We aimed to investigate whether baseline lesion-absorbed dose can predict lesion-based responses and to explore the connection between lesion-absorbed dose and prostate-specific antigen (PSA) response. Methods: In this retrospective study, we evaluated 50 patients with 335 index lesions undergoing 177Lu-PSMA-617 RPT, who had dosimetry analysis performed on SPECT/CT at 24 h after cycles 1 and 2. First, we identified the index lesions for each patient and measured the lesion-based absorbed doses. Lesion-based response was calculated after cycle 2. Additionally, PSA50 response (a decline of 50% from baseline PSA) after cycle 2 was also calculated. The respective responses for mean and maximum absorbed doses and prostate-specific membrane antigen (PSMA) volumetric intensity product (VIP-PSMA) at cycles 1 and 2 were termed SPECTmean, SPECTmaximum, and SPECTVIP-PSMA, respectively. Results: Of the 50 patients reviewed, 46% achieved a PSA50 response after cycle 2. Of the 335 index lesions, 58% were osseous, 32% were lymph nodes, and 10% were soft-tissue metastatic lesions. The SPECT lesion-based responses were higher in PSA responders than in nonresponders (SPECTmean response of 46.8% ± 26.1% vs. 26.2% ± 24.5%, P = 0.007; SPECTmaximum response of 45% ± 25.1% vs. 19% ± 27.0%, P = 0.001; SPECTVIP-PSMA response of 49.2% ± 30.3% vs. 14% ± 34.7%, P = 0.0005). An association was observed between PSA response and SPECTVIP-PSMA response (R2 = 0.40 and P < 0.0001). A limited relationship was found between baseline absorbed dose measured with a 24-h single time point and SPECT lesion-based response (R2 = 0.05, P = 0.001, and R2 = 0.03, P = 0.007, for mean and maximum absorbed doses, respectively). Conclusion: In this retrospective study, quantitative lesion-based response correlated with patient-level PSA response. We observed a limited relationship between baseline absorbed dose and lesion-based responses. Most of the variance in response remains unexplained solely by baseline absorbed dose. Establishment of a dose–response relationship in RPT with a single time point at 24 h presented some limitations.
The relationship between lesion-absorbed dose and tumor response for radiopharmaceutical therapies (RPTs) is not well understood. As RPT fundamentally involves radiation delivery, knowledge of the absorbed dose versus the tumor response in external-beam radiotherapy serves as a useful starting point for evaluating this relationship for RPT. Dose–response relationships have been described in external-beam radiotherapy, aiding in predicting tumor response and potential tissue toxicity (1–3). Moreover, lesion dosimetry has been shown to optimize dose delivery for various cancers, playing a crucial role in treatment outcomes (4–10).
Unlike external-beam radiotherapy, where we have the ability to control and modify the absorbed dose to normal tissues and tumors, nonuniform dose delivery in systemic RPT renders the understanding of dose–response relationship considerably more challenging (11–13). There are preliminary data on dose response with 177Lu-DOTATATE in pancreatic neuroendocrine tumors, demonstrating correlation between tumor-absorbed dose and tumor size reduction (14). The same group was, however, not able to establish a dose–response relationship in small-bowel neuroendocrine tumors (15). Most recently, in patients with gastroenteropancreatic neuroendocrine tumors who underwent 177Lu-DOTATATE therapy, a study demonstrated that the tumor-absorbed dose was associated with radiologic response but not with overall survival (16).
Prostate-specific membrane antigen (PSMA)–targeting RPT using 177Lu-PSMA-617 has demonstrated favorable prostate-specific antigen (PSA) response rates and longer progression-free survival and overall survival in patients with metastatic castration-resistant prostate cancer (17,18). Violet et al. performed whole-body tumor dosimetry and showed a correlation between absorbed tumor doses and PSA response after 177Lu-PSMA-617 RPT at 12 wk (19). Another study looking into index lesion–based dosimetry did not find a significant difference in mean absorbed dose and index lesion dose between PSA responders and nonresponders (20).
Overall, the relationship between lesion-absorbed dose and response in PSMA RPT remains unknown. Therefore, we aimed to analyze whether baseline lesion-absorbed dose, calculated with a single time point measured at 24 h, predicts lesion-based response in patients treated with 177Lu-PSMA-617 RPT. Additionally, we analyzed the relationship between response based on lesion-absorbed dose and PSA response.
MATERIALS AND METHODS
Study Population
We conducted a retrospective review of patients with metastatic castration-resistant prostate cancer who received 177Lu-PSMA-617 RPT between June 2022 and January 2023 at our institution. All patients who received a minimum of 2 cycles, with a treatment interval of 6–8 wk, and 24-h posttherapy SPECT/CT were included. The institutional review board approved this retrospective study, and the requirement to obtain informed consent was waived.
Posttherapy Scan Acquisition
Whole-body planar and SPECT/CT imaging were performed 1 d after each cycle in the context of routine clinical care using a dual-head γ-camera (Infinia Hawkeye; GE Healthcare) with the following acquisition parameters: 208% ± 10% keV photopeak, 170% ± 10% keV scatter window, 128 × 128 matrix, 20–30 s per projection, 60 projections in total using 2 detectors, medium-energy general-purpose collimators, and a low-dose CT scan for attenuation correction.
Lesion Selection
For each patient, lesions in each of 3 regions were included: lymph node, osseous, and soft tissue (e.g., liver, lung or adrenals). We included the highest- and lowest-expressing lesion based on visual assessment within each region, and the 3 largest remaining lesions, for up to a total of 5 lesions per region. To be included, soft-tissue lesions had to measure more than 10 mm along the long axis and lymph nodes had to measure more than 15 mm along the short axis on the CT component of the SPECT/CT scan. Osseous lesions had to measure more than 2 cm3 in SPECT-segmented volume in order minimize the effect of partial-volume averaging (21–24). For liver and lung lesions, individual lesions were segmented individually, although if confluent, adjacent lesions were grouped.
Absorbed Dose Calculation
MIM SPECTRA Quant (MIM Software) was used for image reconstruction with an iterative ordered-subset expectation-maximization algorithm using 4 iterations and 10 subsets. Scatter correction, attenuation correction, and resolution recovery were applied (25). The kidneys were segmented using the autocontouring software from MIM with manual adjustment to region of interest as required. Quantitative dosimetry measurements were performed on the index lesions and kidneys at cycles 1 and 2. Lesion-based segmentation was done by a nuclear medicine physician using a standardized semiautomated workflow using SurePlan MRT (MIM software), which applies a single imaging time point for biokinetic evaluation based on the Hänscheid approach and performs dosimetry using voxel-based absorbed dose calculation using voxel S-value (26,27). To determine mean and maximum absorbed doses in grays, we performed segmentation on the dose map using a 42% threshold of the maximum (26,28). Additionally, similar to tumor lesion glycolysis and volumetric intensity product (VIP) used previously (16,29), we calculated the VIP-PSMA by multiplying the tumor volume and mean absorbed dose of the index lesions, excluding the least avid lesion.
Lesion-Based Response Evaluation
Mean and maximum absorbed doses and VIP-PSMA at cycles 1 and 2 were compared and their respective responses (termed SPECTmean, SPECTmaximum, and SPECTVIP-PSMA, respectively) were calculated using the following formula:
Biochemical Response Evaluation
Serum PSA levels served as the standard of reference for response assessment to therapy (30,31). Serum PSA from the day of the first cycle and 3 wk after the second cycle was used to calculate PSA response. A decline of 50% from baseline PSA was defined as a PSA50 response, and accordingly, patients were categorized as responders or nonresponders.
Radiographic Response Evaluation
Radiographic response was evaluated on the CT component of SPECT/CT scans for both cycle 1 and cycle 2. For lymph nodes, the maximum of the small-axis diameter was measured, whereas for other soft-tissue lesions, the maximum of the long-axis diameter was measured. Although lesion-level analysis was being performed, the relative change in the lesion size was calculated using the following equation, where D1 represents the lesion diameter at cycle 1 and D2 corresponds to the diameter at cycle 2: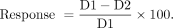
Statistical Analysis
Descriptive statistics in the form of median with interquartile range and mean with SD for continuous variables and count with percentage for binary variables were used to describe quantitative variables from the clinical data. Lesion-level data were averaged for each patient to obtain patient-level data, and results were expressed as mean with SD. A Student t test was conducted to assess the relationship between lesion-based response and PSA response between responders and nonresponders. We used a linear regression model for patient-level analysis to assess the association between various predictors (baseline mean and maximum absorbed dose and VIP-PSMA) and PSA response. We used a linear mixed model for lesion-level analysis to explore the association between different parameters of absorbed dose and lesion-based response while considering that the data from the same subject share the same random component. Of the 5 lesions segmented for each patient, 4 lesions excluding the least avid lesions were termed index lesions and were analyzed collectively. The results for the least avid index lesions were analyzed separately. Because of lack of an established framework for SPECT lesion-based response, we extrapolated from the 30% decrease used in PERCIST and RECIP (32,33). A P value of less than 0.05 was considered significant.
RESULTS
Patient Characteristics
Fifty-seven sequential patients with metastatic castration-resistant prostate cancer received 177Lu-PSMA RPT from June 2022 to January 2023. Seven patients did not meet the inclusion criteria. A patient flowchart and the demographic data are provided in Table 1 and Supplemental Figure 1. Of the 50 patients analyzed, 23 (46%) achieved a PSA50 response after cycle 2. Of the 335 index lesions analyzed, 58% (194) were osseous, 32% (107) were lymph nodes, and 10% (34) were soft-tissue lesions. Two patients were low PSA secretors and were excluded from the PSA response analysis. At the patient level, the average of the mean absorbed dose at cycle 1 was 7.5 ± 6.2 Gy. At the lesion level, the average of the mean absorbed dose at cycle 1 was 6.7 ± 6.6 Gy (Fig. 1). The mean absorbed dose to kidneys during cycles 1 and 2 was 1.1 ± 0.2 Gy and 1.1 ± 0.5 Gy, respectively (Table 1).
Patient Demographics and Dosimetry Data
Scatterplots showing distribution of lesion-absorbed doses at cycle 1. (A) Mean of lesion mean absorbed dose within each individual patient, with whiskers representing SD. (B) Mean absorbed dose for each individual index lesion, with whiskers representing SD of dose within individual lesion.
Lesion-Based Response and PSA Response
The SPECTmean response from cycle 1 to cycle 2 was 46.8% ± 26.1% for responders and 26.2% ± 24.5% for nonresponders (P = 0.007). The SPECTmaximum response from cycle 1 to cycle 2 was 45% ± 25.1% for responders and 19% ± 27.0% for nonresponders (P = 0.001). The SPECTVIP-PSMA response from cycle 1 to cycle 2 was 49.2% ± 30.3% for responders and 14% ± 34.7% for nonresponders (P = 0.0005, Fig. 2). There was an association between PSA response and SPECTVIP-PSMA response for the index lesions and for the single most avid lesion (R2 = 0.40 and P < 0.0001 for index lesions and R2 = 0.35 and P < 0.0001 for the single most avid lesion, respectively).
SPECTmean response (A), SPECTmaximum response (B), and SPECTVIP-PSMA response (C) in PSA50 responders and nonresponders. Box plots display median and first and third quartiles. One outlier was observed at −100% for SPECTVIP-PSMA response.
Lesion-Level Radiographic Response and SPECT-Based Response
For the lymph nodes, there was an association between radiographic response and SPECT-based response for the index lesions (R2 = 0.58 and P < 0.0001 for SPECTmean response, R2 = 0.67 and P < 0.0001 for SPECTmaximum response, and R2 = 0.42 and P < 0.0001 for SPECTVIP-PSMA response). For the soft tissues, among all the parameters, SPECTVIP-PSMA response for the index lesions had an association with the radiographic response (R2 = 0.34 and P < 0.005). Additionally, for both the soft tissues and the lymph nodes, no association was observed between the baseline lesion-absorbed dose and the lesion-level radiographic response.
Baseline Lesion-Absorbed Dose and PSA Response
The mean lesion-absorbed dose at cycle 1 was 8.1 ± 6.7 Gy for responders and 7.3 ± 5.9 Gy for nonresponders (P = 0.7). The maximum lesion-absorbed dose during cycle 1 was 15.8 ± 13.0 Gy for responders and 14.0 ± 11.8 Gy for nonresponders (P = 0.6). The VIP-PSMA during cycle 1 was 104.9 ± 238.0 for responders and 61.0 ± 74.6 for nonresponders (P = 0.3). No association was observed between the baseline lesion-absorbed dose and PSA response (Supplemental Table 1).
Baseline Lesion-Absorbed Dose and Lesion-Based Response
With the 30% reduction response criterion, the mean absorbed dose during cycle 1 was 7.7 ± 8.3 Gy in responders (n = 30) and 7.3 ± 4.6 Gy in nonresponders (n = 20) (P = 0.8). The maximum absorbed dose during cycle 1 was 14.9 ± 10.6 Gy in responders and 14.1 ± 13.8 Gy in nonresponders (P = 0.8). The VIP-PSMA during cycle 1 was 113.4 ± 240.0 in responders and 59.1 ± 72.7 in nonresponders (P = 0.2). With a linear mixed-effects model at the lesion level, there was a minimal relationship between baseline absorbed dose and lesion-based response (R2 = 0.05, P = 0.001, for mean absorbed dose and SPECTmean response; R2 = 0.03, P = 0.007, for maximum absorbed dose and SPECTmaximum response; Supplemental Table 1).
Location of Disease and Response
Lymph nodes had a higher mean absorbed dose than did osseous metastases during cycle 1 (8.3 Gy ± 9.4 Gy and 5.9 ± 4.5 Gy, respectively, P = 0.001). However, no significant difference was noted in the lesion-based response between lymph nodes and osseous metastases (SPECTmean response of 36.9% vs. 33.7%, P > 0.05). The mean absorbed dose during cycle 1 was the lowest for soft-tissue metastases (5.6 ± 4.1 Gy), and there was no significant difference between the lesion-based response in soft-tissue metastases and in the other 2 metastatic sites (SPECTmean response of 39%, P > 0.05, Supplemental Table 2).
Least Avid Lesions
There were 81 least avid lesions included. Although the mean absorbed dose during cycle 1 was significantly lower in the least avid lesions (3.68 ± 4.2 Gy, P < 0.001), there was no significant difference in lesion-based response between the least avid and other index lesions (SPECTmean of 30% vs. 36.5%, P > 0.05).
DISCUSSION
Our results suggest that patients with a PSA50 after 2 RPT cycles showed a significantly better SPECT/CT lesion-based response than did nonresponders. For the index lesions, neither the lesion-based response nor the PSA response strongly correlated with the baseline absorbed dose obtained using single SPECT imaging at 24 h.
In routine clinical practice, it is common to perform 24-h 177Lu SPECT/CT scans because of their practicality. Since these were the only quantitative data available, we performed single-time-point dosimetry using the Hänscheid method despite its not being the optimal time point for dosimetric analysis as indicated by existing literature (27,34–36). The Hänscheid method, particularly when applied at the 24-h time point, underestimates absorbed doses to both the kidneys and the tumors (35–37). Consequently, our computed absorbed doses were comparatively lower than those reported in the literature (19,20). Although the reported doses are underestimated, the relative dose across patients and between patients at cycles 1 and 2 are reflective of the effect of the absorbed dose.
We did not observe a relationship between baseline lesion-absorbed dose and PSA response. Note that we looked at only 5 index lesions and not the total tumor burden. Violet et al. found higher whole-body tumor doses in patients with a PSA50, but index lesion dose did not correlate with PSA50 (19). Of note, they compared index lesion–absorbed dose with patient-level response and not with individual lesion-level response. Similarly, our results did not show a relationship between baseline lesion-absorbed dose and PSA response. Völter et al. also failed to demonstrate a relationship between index lesion–absorbed dose and PSA response (20). Conversely, a study investigating RPT in patients with low-volume hormone-sensitive metastatic prostate cancer found a statistically significant correlation between the absorbed dose to the index lesion and PSA response, although in only 10 patients (38). We also observed an absorbed dose to the kidneys that would result in a lower absorbed dose than the 23 Gy to the kidneys extrapolated from external-beam radiation therapy. It could be that the lack of a dose–response relationship seen in our cohort is because the absorbed doses do not yet meet a threshold for tumor response; that is, we may be currently underdosing patients. Studies in the future that escalate the administered activity on the basis of the limits of target organs may result in more robust dose–response relationship. Dose escalation studies aiming to reach toxicity levels may establish a dose–response relationship in RPTs that has not yet been demonstrated in the literature.
Although we found a statistically significant relationship between baseline mean and maximum absorbed dose and their respective SPECTmean and SPECTmaximum responses, the baseline absorbed dose is not sufficient to explain the variation in SPECT lesion-based response, suggesting that additional variables such as the tumor microenvironment should also be taken into account. Larger solid tumors with necrotic cores might have larger doses deposited in the necrotic region and would require a higher dose in the periphery (39). The tumor microenvironment, particularly the cancer-associated fibroblasts and the secretory factors, contribute to radiation resistance through signaling and immune modulation (40). Lastly, intrinsic radiation sensitivity is influenced by DNA repair, apoptosis, cellular proliferation, and hypoxia (41–43). Factors affecting tumor-absorbed dose and response in RPT are not fully understood, and ongoing analyses are aimed at shedding light on these aspects (44,45).
A recent study showed that 41.4% of the interviewed physicians made decisions to discontinue RPT based on clinical progression alone, signifying that the optimal measure for response assessment in RPT is still unclear (46). PSA as an early marker of response is not always accurate. Assessing measurable disease in radiographic response assessment on CT is hindered by bone metastases (47,48). PSMA PET–based parameters have been suggested (33,49,50), but their clinical implementation has yet to be seen.
Our results suggest that the SPECTmean, SPECTmaximum, and SPECTVIP-PSMA responses were significantly higher in PSA responders than nonresponders. A 40% association between SPECTVIP-PSMA response and PSA response was noted in our study, whereas this association was less than 10% for baseline absorbed dose and lesion-based response. Additionally, we also demonstrated that SPECTVIP-PSMA response correlated with radiographic response for lymph nodes and soft-tissue metastases. Although baseline absorbed dose did not correlate with subsequent response, the relative change in the absorbed dose over cycles does match PSA and radiographic response well. The change in dose over cycles likely indicates the radiation sensitivity of the tumor. In parallel with our quantitative lesion-based dosimetric analysis, John et al. pursued a qualitative approach to assess response to RPT using SPECT/CT imaging assessment (51). SPECT/CT-based analysis, in terms of both qualitative and quantitative parameters, is an efficient and practical tool for response assessment to RPT.
One of the main limitations of this study is the use of single-time-point dosimetry at 24 h using the Hänscheid method. More accurate dosimetry with multiple time points or later time points may be helpful to understand the relationship between dose and response. Moreover, tumors usually present uptake and washout phases (i.e., a biexponential behavior) that is not correctly modeled by the Hänscheid simplification (27).
There are other limitations to our study as well. First, we did not perform radiographic or PSMA PET–based response evaluation to assess the dose–response relationship. Second, we did not perform whole-body tumor dosimetry analysis, which may have yielded different results; however, our goal was to understand relationships between dose and response at the lesion level. Third, the impact of additional treatment cycles and cumulative activity on the PSA outcomes for our patient group remains unclear because we focused on response with the first 2 cycles.
CONCLUSION
Establishing dose–response relationship in RPT remains challenging. Quantitative dosimetry lesion-based analysis of SPECT/CT imaging at cycles 1 and 2 of RPT predicted a better lesion-based response in PSA responders. Patient with higher lesion-based responses on cycle 2 dosimetry had a higher chance of PSA response. Although we observed a significant relationship between baseline absorbed dose and lesion-based responses, most of the variance in response remains unexplained by baseline absorbed dose alone. Lesion-based response on SPECT/CT in combination with PSA response may serve as an early response marker to 177Lu-PSMA RPT.
DISCLOSURE
Thomas Hope has grant funding to the institution from Clovis Oncology, GE Healthcare, Lantheus, Janssen, the Prostate Cancer Foundation, Telix Pharmaceuticals, and the National Cancer Institute (R01CA235741 and R01CA212148). He received personal fees from Bayer, Cardinal Health, BlueEarth Diagnostics, and Lantheus and received fees from and has an equity interest in RayzeBio and Curium. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the relationship between baseline lesion-absorbed dose and lesion-based response in 177Lu-PSMA-617 RPT, and how does this correlate with PSA response?
PERTINENT FINDINGS: PSA50 responders exhibited a significantly better lesion-based response on SPECT/CT after cycle 2 than did nonresponders. Although there was a relationship between baseline absorbed dose and lesion-based response, most of the variance cannot be attributed solely to baseline absorbed dose.
IMPLICATIONS FOR PATIENT CARE: Incorporating lesion-based response evaluation on SPECT/CT imaging with PSA response may be a valuable early response marker for 177Lu-PSMA RPT.
Footnotes
Published online May 9, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 7, 2023.
- Accepted for publication April 8, 2024.










