Visual Abstract
Abstract
Anti–programmed death 1 (PD-1) inhibitors are the standard of care for advanced gastroesophageal cancer. Although recommendations and approval by regulatory agencies are often based on programmed death ligand 1 (PD-L1) expression, pathologic assessments of PD-L1 status have several limitations. Single-site biopsies do not adequately capture disease heterogeneity within individual tumor lesions or among several lesions within the same patient, the PD-L1 combined positive score is a dynamic biomarker subject to evolution throughout a patient’s disease course, and repeated biopsies are invasive and not always feasible. Methods: This was a prospective pilot study of the PD-L1–targeting radiotracer, 18F-BMS-986229, with PET imaging (PD-L1 PET) in patients with gastroesophageal cancer. Patients were administered the 18F-BMS-986229 radiotracer intravenously at a dose of 370 MBq over 1–2 min and underwent whole-body PET/CT imaging 60 min later. The primary objective of this study was to evaluate the safety and feasibility of 18F-BMS-986229. The trial is registered with ClinicalTrials.gov (NCT04161781). Results: Between February 3, 2020, and February 2, 2022, 10 patients with gastroesophageal adenocarcinoma underwent PD-L1 PET. There were no adverse events associated with the 18F-BMS-986229 tracer, and imaging did not result in treatment delays; the primary endpoint was achieved. Radiographic evaluation of PD-L1 expression was concordant with pathologic assessment in 88% of biopsied lesions, and 18F-BMS-986229 uptake on PET imaging correlated with pathologic evaluation by the combined positive score (Spearman rank correlation coefficient, 0.64). Seventy-one percent of patients with 18F-BMS-986229 accumulation on PET imaging also had lesions without 18F-BMS-986229 uptake, highlighting the intrapatient heterogeneity of PD-L1 expression. Patients treated with frontline programmed death 1 inhibitors who had 18F-BMS-986229 accumulation in any lesions on PET imaging had longer progression-free survival than patients without tracer accumulation in any lesions (median progression-free survival, 28.4 vs. 9.9 mo), though the small sample size prevents any definitive conclusions. Conclusion: PD-L1 PET imaging was safe, feasible, and concordant with pathologic evaluation and offers a potential noninvasive tool to assess PD-L1 expression.
With 1.3 million deaths annually, gastroesophageal cancer (GEC) represents the second leading cause of cancer-related death globally (1). In the past 2 y, programmed death 1 (PD-1) inhibitors in combination with chemotherapy have become the standard of care in the frontline metastatic setting, with regulatory approval and patient selection often based on programmed death ligand 1 (PD-L1) expression (2–4). Although a high PD-L1 combined positive score (CPS) has been associated with better outcomes, pathologic assessment has several limitations. Single-site biopsies do not adequately capture disease heterogeneity within individual tumor lesions or among several lesions within the same patient, PD-L1 CPS is a dynamic biomarker subject to evolution throughout a patient’s disease course, and repeated biopsies are invasive and not always feasible. Furthermore, pathologic assessment is operator-dependent and can be influenced by the choice of PD-L1 immunohistochemical assay, tumor content, and the quality of fixation (5–7). Although these technical limitations are minimized in large phase 3 studies, discordant results are more common in routine clinical practice (8). Therefore, there is a need for comprehensive, less invasive evaluation of PD-L1 expression in GEC.
Given the similar challenges in evaluating human epidermal growth factor receptor 2 positivity, the fact that the human epidermal growth factor receptor 2–targeted tracer 89Zr-trastuzumab has been successful in identifying human epidermal growth factor receptor 2 positivity in GEC suggests that a similar approach may be useful to assess PD-L1 status (9,10). Radiolabeled PD-1 and PD-L1 antibodies have been evaluated in patients with non–small cell lung cancer, bladder cancer, and triple-negative breast cancer (11–13). However, because of slower kinetics, radiolabeled antibodies require that imaging be performed several days to a week after tracer injection. Lower-molecular-weight PD-L1 tracers allow for same-day injection and imaging. 18F-BMS-986192, which was evaluated in non–small cell lung cancer and melanoma, demonstrated a correlation with pathologic evaluation and responses using this method but was challenging to synthesize and was isolated in only modest radiochemical yields (13,14). 18F-BMS-986229 is a macrocyclic peptide with high affinity for PD-L1, tight binding with a slow off-rate from the receptor, rapid clearance from non–PD-L1–expressing tissues, and the ability to be isolated in higher yields while also being less challenging to synthesize than 18F-BMS-986192 (15,16). Preclinical evaluations of 18F-BMS-986229 demonstrated specific binding to PD-L1–expressing tissues in vitro and in vivo (15,16). We report what is, to our knowledge, the first study of the PD-L1–targeting radiotracer18F-BMS-986229 with PET imaging (PD-L1 PET) performed on patients with GEC and the first clinical use of 18F-BMS-986229 as a PD-L1–targeting radiotracer.
MATERIALS AND METHODS
Patients and Study Design
This was a single-institution prospective pilot open-label microdose PET evaluating the PD-L1–targeting radiotracer 18F-BMS-986229 with PET imaging in 10 patients with GEC between February 3, 2020 and February 2, 2022. Ten (100%) patients had adenocarcinoma, 7 (70%) had metastatic disease, and 3 (30%) had received prior treatment at the time of PD-L1 PET imaging, including 2 with PD-1 inhibitors (Table 1). The median PD-L1 CPS was 10 (interquartile range, 5–20). All 10 patients underwent PD-L1 PET imaging, and 1 patient underwent repeat PD-L1 PET after 3 cycles of nivolumab.
Baseline Demographic and Clinical Characteristics
The study protocol was approved by Memorial Sloan Kettering Cancer Center’s institutional review board and ethics committees, and all subjects signed an informed consent form. The trial is registered with ClinicalTrials.gov (NCT04161781). Eligible patients were aged 18 y or older with a diagnosis of esophageal, gastric, or gastroesophageal junction adenocarcinoma or esophageal squamous cell carcinoma and a PD-L1 CPS of at least 1 as reviewed by a Memorial Sloan Kettering Cancer Center pathologist. PD-L1 immunohistochemistry was performed using clone E1L3N (Cell Signaling Technology) as per standard Memorial Sloan Kettering Cancer Center practice. The PD-L1 CPS was defined as the number of PD-L1–positive tumor cells, lymphocytes, and macrophages divided by the total number of viable tumor cells multiplied by 100. Patients were not limited by stage of disease and were permitted to undergo PD-L1 PET imaging at any time during their treatment course. Additional key inclusion criteria included radiographically measurable or evaluable disease as per RECIST 1.1, an Eastern Cooperative Oncology Group performance status of 2 or better, and adequate organ function. Patients with autoimmune diseases or immunodeficiencies and those on steroids or other immunosuppressive therapies were excluded.
All patients were administered the 18F-BMS-986229 radiotracer intravenously at a dose of 370 MBq over 1–2 min and underwent whole-body PET/CT imaging 60 min (range, 55–70 min) later. PET/CT imaging took 30 min, and the patients were observed for an additional 30 min after the scan, for a total of 120 min after injection of the radiotracer. The patients were permitted to undergo an optional repeat PD-L1 PET after 6 wk of anti–PD-1 therapy.
The primary objective of the study was to evaluate the safety and feasibility of 18F-BMS-986229. PD-L1 PET imaging would be considered safe and feasible if there were no grade 3 or higher 18F-BMS-986229–related adverse events and if at least 70% of patients were found to be PD-L1 PET–positive. Safety was assessed in all patients who received 18F-BMS-986229. Toxicity and adverse events were assessed according to the National Cancer Institute’s common terminology criteria for adverse events, version 5.0. Safety follow-up was conducted via phone within 14–21 d after the injection. The secondary objectives included comparing PD-L1 PET imaging findings with 18F-FDG PET/CT, CT, and PD-L1 CPS by immunohistochemistry.
18F-BMS-986229 Drug Product
The radiolabeling precursor was obtained from Bristol Myers Squibb Inc. by Memorial Sloan Kettering Cancer Center’s Radiochemistry and Molecular Imaging Probes Core Facility in compliance with the Food and Drug Administration investigational new drug application. 18F-BMS-986229 was designed using BMS-986189, a potent macrocyclic peptide–derived PD-L1 antagonist with picomolar PD-L1 affinity as the starting point. On the basis of the cocrystal structure of BMS-986189 and PD-L1, a propargyl glycine moiety was incorporated into the portion of the peptide that was solvent-exposed. This macrocyclic peptide was labeled with 18F via a covalent bond by the use of the copper-catalyzed azide–alkyne cycloaddition reaction with a propargyl glycine on the peptide and an azide-containing 18F-prosthetic group, 18F-BMT-187144 (15).
Imaging
Each patient underwent whole-body PET/CT from mid skull to proximal thigh. All scans were obtained using a Discovery 710 PET/CT scanner (GE Healthcare) in 3-dimensional mode with attenuation, scatter, and other standard corrections applied and using iterative reconstruction. Patients also underwent dedicated CT of the chest, abdomen, and pelvis and whole-body 18F-FDG PET as a reference standard.
Images were analyzed for tracer distribution in normal body tissues and within RECIST-measurable target and nontarget lesions as well as 18F-FDG PET–avid lesions. Analysis was performed by an experienced nuclear medicine physician who was aware of the patient’s history and conventional imaging results. Localization in the tumor was defined as a focal accumulation greater than adjacent background uptake in areas in which physiologic activity was not expected. The maximum uptake in each individual tumor lesion, confirmed on CT or 18F-FDG PET, was measured.
Definition of PD-L1 PET Avidity
Active lesions were those that were positive on 18F-FDG PET/CT or at least 2 cm in size on dedicated CT. Active lesions were graded on PD-L1 PET on a score of 1 to 5, with scores of 4 or 5 considered positive and PD-L1 PET–avid. A score of 1 indicated no uptake and was denoted as negative. A score of 2 indicated uptake less than the adjacent background uptake and was denoted as probably negative. A score of 3 indicated uptake greater than adjacent background uptake but possibly normal physiologic uptake and was denoted as indeterminate. A score of 4 indicated uptake moderately greater than adjacent background uptake and unlikely to be normal physiologic uptake and was denoted as probably positive. A score of 5 indicated uptake markedly greater than adjacent background uptake and clearly not normal physiologic uptake and was denoted as positive. A patient was considered to have a positive PD-L1 PET result if at least 25% of active lesions were positive.
Statistical Analysis
PD-L1 PET imaging results, including visualization score, as well as SUVmax and SUVmean among all lesions within a patient, were correlated with the PD-L1 CPS using the Spearman rank correlation coefficient (rs). Clinical outcomes were reported using descriptive statistics given the small sample size.
RESULTS
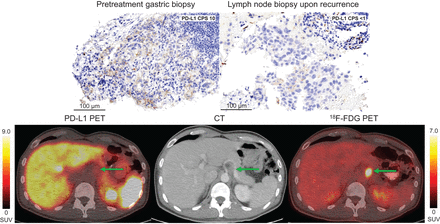
There were no adverse events associated with the 18F-BMS-986229 tracer. Imaging did not result in treatment delays, and the study met the primary safety and feasibility endpoint. Seven of 8 (88%) patients in whom the biopsied lesion was radiographically evaluable had PD-L1 PET tracer uptake, concordant with PD-L1 CPS (Fig. 1A). Two patients had sites biopsied that were not radiographically evaluable because of prior intervening treatment. Notably, both patients had other disease sites that were PD-L1 PET–avid (Fig. 1B). Although a PD-L1 CPS of 1 or higher in any tumor biopsy was required for inclusion in the study, 2 patients who had a PD-L1 CPS of 1 or higher on an initial biopsy had a subsequent biopsy, still before PD-L1 PET imaging, that showed a PD-L1 CPS of less than 1. Both lesions that had a PD-L1 CPS of less than 1 appropriately did not have uptake on PD-L1 PET (Fig. 2).
Concordance between PD-L1 PET, PD-L1 CPS, and 18F-FDG PET. (A) PD-L1 pathologic evaluation by CPS correlated with PD-L1 PET visualization score (rs = 0.64) and SUV (rs = 0.61). (B) All 10 patients are plotted according to their maximum PD-L1 PET visualization score. Visualization score of 4 or 5 is considered positive. (C) Among 8 patients with PD-L1 CPS–positive tumors, there were 21 18F-FDG–avid lesions, of which 9 were PD-L1 PET–avid. Total numbers of PD-L1 PET–avid lesions and 18F-FDG PET–avid lesions at each disease site are shown, as well as those that were 18F-FDG–avid but not PD-L1–avid.
PD-L1 PET without uptake corresponds to PD-L1–negative biopsy. Gastric mass biopsy (top left) demonstrated PD-L1 CPS of 10. Patient was treated with chemotherapy followed by surgery but then developed metastatic recurrence to lymph nodes, biopsy of which demonstrated PD-L1 CPS < 1 (top right). PD-L1 PET (bottom left), corresponding CT (bottom middle), and 18F-FDG PET (bottom right) obtained at time of recurrence depict non–PD-L1–avid but 18F-FDG–avid gastrohepatic lymph node (arrows) that was biopsied. Left intensity scale bar corresponds to PD-L1 PET, and right color scale bar corresponds to 18F-FDG PET. Both are in absolute SUV units.
Between the PD-L1 CPS and PD-L1 PET visualization score, rs was 0.64, and between the PD-L1 CPS and PD-L1 PET SUV, rs was 0.61. Those with higher PD-L1 PET visualization scores had a numerically higher PD-L1 CPS. Patients with a maximum PD-L1 PET visualization score of 5 of 5 (n = 4) had a median PD-L1 CPS of 22.5 (range, 5–70), whereas those with a maximum PD-L1 PET visualization score of 4 (n = 2) had a PD-L1 CPS of 5 and 20, and those without any PD-L1 PET–avid lesions (visualization score of 3 or less; n = 4) had a median PD-L1 CPS of 5 (range, 0–10). Among the 8 patients with PD-L1 CPS–positive tumors, there were 21 18F-FDG PET–avid lesions (Fig. 1C). Patients did not receive any treatment between PD-L1 PET and 18F-FDG PET imaging, and scans occurred within a median of 7 d of each other. Nine of these lesions had uptake on PD-L1 PET imaging. PD-L1 PET avidity coincided with 18F-FDG PET avidity at the primary tumor, as well as bone, lung, and adrenal metastases, whereas most PD-L1 PET lesions without uptake were lymph nodes.
PD-L1 PET highlighted PD-L1 spatial heterogeneity; 5 of the 7 patients with PD-L1 PET–avid lesions also had sites of disease without tracer accumulation, 4 of whom had a greater than 2-fold difference in SUV between the most avid and least avid lesions (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). One of the 2 patients with uptake across all disease sites developed progression after 5 cycles of frontline 5-fluorouracil and oxaliplatin (FOLFOX) chemotherapy, was then treated with pembrolizumab monotherapy, achieved a durable partial response, and continued pembrolizumab for 10.9 mo before ultimately developing progression of disease (Fig. 3A). By contrast, one of the patients with heterogeneous uptake was also treated with frontline FOLFOX followed by second-line pembrolizumab but developed progression of disease after just 2 mo.
PD-L1 PET tracer accumulation across multiple lesions and in response to treatment. (A) PD-L1 PET imaging demonstrating tracer accumulation across both adrenal (top left) and vertebral (bottom left) lesions in patient 6, who had primary progression on FOLFOX chemotherapy after 2 mo but durable (10.9 mo) response to pembrolizumab as second-line therapy. Corresponding CT and 18F-FDG PET scans obtained at same time as PD-L1 PET are shown in middle and right panels, respectively. (B) Patient 8 with locally advanced, unresectable gastroesophageal junction (GEJ) adenocarcinoma had tracer accumulation at primary GEJ mass (left) and was treated with FOLFOX and nivolumab. He had significant tumor regression (right) allowing for surgical resection, received adjuvant 5-fluorouracil (5-FU) and nivolumab, and remained without evidence of disease 21.9 mo later. Left intensity scale bar corresponds to both PD-L1 and 18F-FDG PET images in Figure 3A. Right intensity scale bar corresponds to both PD-L1 PET images in Figure 3B.
Seven patients had untreated, advanced disease at the time of PD-L1 PET imaging, 6 of whom were treated with PD-1 inhibitors with or without chemotherapy. Median follow-up among living patients was 25.6 mo. Three had lesions with uptake on PD-L1 PET imaging: 2 achieved a radiographic complete response with progression-free survival (PFS) of 31.6 and 28.4 mo, and the third achieved a partial response. The third patient underwent repeat PD-L1 PET imaging after 3 cycles of FOLFOX with nivolumab, and significant tumor regression was seen, allowing for surgical resection. He remained without any evidence of disease 21.9 mo later (Fig. 3B). Among the 3 patients with negative PD-L1 PET imaging, 1 achieved a complete response (PFS, 14.2 mo), 1 achieved a partial response (PFS, 9.9 mo), and 1 had progressive disease as best response (PFS, 2.0 mo).
DISCUSSION
To our knowledge, this study was the first to evaluate PD-L1 PET imaging in patients with GEC. The study demonstrated that same-day imaging with the PD-L1–targeting radiotracer 18F-BMS-986229 is safe, feasible, and concordant with pathologic assessments of PD-L1 expression. PD-L1 PET imaging also highlighted spatial heterogeneity across lesions within individual patients and appeared to be associated with outcomes, as patients with uptake had better responses and numerically longer PFS when treated with PD-1 inhibitors than did patients without tracer accumulation at any sites of disease. The small sample size limits any definitive conclusions, though these results provide evidence upon which larger studies could be conducted.
Importantly, PET imaging with 18F-BMS-986229 may be performed 1 h after tracer administration. This is in contrast to radiolabeled antibodies that require separate clinic visits several days apart (11–13). The ability to perform same-day imaging and its convenience for patients are crucial to enabling broad implementation. 18F-BMS-986192, another low-molecular-weight PD-L1–targeting radiotracer that allows for same-day imaging, has been evaluated in patients with non–small cell lung cancer and melanoma; however, 18F-BMS-986229 is more easily synthesized and may be isolated in higher yields, although the two have never been directly compared clinically (13–16).
The study was limited by its small sample size and the heterogeneous clinical context in which patients underwent PD-L1 PET imaging, namely that some patients had untreated, locally advanced tumors whereas others had metastatic, previously treated disease. In future trials it will be important to obtain baseline and on-treatment PD-L1 PET scans to determine what metrics best predict for long-term outcomes and response to PD-1 and PD-L1 inhibitors, such as maximum avidity, homogeneous avidity, or changes in avidity, as has been demonstrated previously with 18F-FDG PET, and to validate radiographic and pathologic concordance in a larger cohort (17). Nevertheless, the study demonstrated that PD-L1 PET imaging has the potential to be a useful tool to assess PD-L1 expression and aid in the management of patients with GEC.
CONCLUSION
PD-L1 PET imaging was safe, feasible, and concordant with pathologic evaluation and demonstrated its potential use as a noninvasive tool to assess PD-L1 expression.
DISCLOSURE
This research was supported by Bristol Myers Squibb, Inc., and the Radiochemistry and Molecular Imaging Probe Core of MSK, supported by NIH/NCI Cancer Center support grant P30 CA008748. Samuel Cytryn previously held equity in Pfizer, Moderna, and BioNTech. Neeta Pandit-Taskar has received research funding from Bayer Health, Bristol Myers Squibb, Clarity Pharmaceuticals, ImaginAb, Janssen, and Regeneron and has served in consulting or advisory roles for Illumina, Progenics, Actinium, Fusion Pharmaceuticals Inc., ImaginAb, and Y-mAbs Therapeutics Inc. Steven Maron has received research funding from Guardant Health (Inst) and Roche/Genentech (Inst); has served in consulting or advisory roles for Amgen, Basilea, Clinical Care Options, Daiichi Sankyo, Elevaton Oncology Inc., Health Advances, MedPage Today LLC, Natera, Novartis, Physicians’ Education Resource, Pinetree Therapeutics Inc., Purple Biotech Ltd., and Vindico Medical Education; and has equity in Calithera Biosciences and McKesson. Geoffrey Ku has served in consulting or advisory roles for AstraZeneca, Bristol Myers Squibb, Merck and Co Inc., and Zymeworks Inc. Serge Lyashchenko has served in consulting or advisory roles for International Atomic Energy Agency and Y-mAbs Therapeutics Inc. and has equity in Evergreen Theragnostics. Jason Lewis has served in consulting or advisory roles for Alpha-9 Theranostics Inc., CSRA Inc., Earli Inc., Elsevier, Inhibrx Inc., The Journal of Nuclear Medicine, Nextech Venture LTD, TPG Capital, and World Molecular Imaging Society; has equity in Alpha-9 Theranostics, Clarity Pharmaceuticals, Evergreen Theranostics, Suba Therapeutics Inc., Summit Biomedical Imaging LLC, Telix Pharmaceuticals Ltd., Trace-Ability Inc., and pHLIP Inc.; and has intellectual property rights in CheMatech, Daiichi Sankyo, Diaprost AB, Elucida Oncology, Samus Therapeutics LLC, and Theragnostics Ltd. Viktoriya Paroder has served in consulting or advisory roles for Gerson Lehrman Group and Medcase Health. Amitabh Srivastava has served on advisory boards or in consulting roles for PathAI Inc. Yelena Janjigian has received research funding from AstraZeneca, Acrus Biosciences, Bayer, Bristol Myers Squibb, Eli Lilly, Roche/Genentech, Inspirna, Merck and Co Inc., and Transcenta and has served in consulting or advisory roles for Abbvie, Amerisource Bergen, Ask-Gene Pharma Inc., Arcus Biosciences, Astellas, AstraZeneca, Basilea Pharmaceutica, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Geneos Therapeutics, GlaxoSmithKline, Guardant Health Inc., Imedex, Imugene, Inspirna, Lynx Health, Merck and Co Inc., Mersana Therapeutics, PeerView Institute, Pfizer, Seagen, Silverback Therapeutics, and Zymerworks Inc. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is PD-L1 PET a safe and effective tool for noninvasive assessment of PD-L1 expression in patients with GEC?
PERTINENT FINDINGS: In a pilot study of 10 patients with GEC, same-day PET imaging with the PD-L1–targeting radiotracer 18F-BMS-986229 was safe and did not lead to any adverse events or delays in treatment. Radiographic assessment of PD-L1 expression was concordant with pathologic assessment by PD-L1 CPS (rs = 0.64). PD-L1 PET also highlighted the heterogeneity of PD-L1 expression and was associated with favorable outcomes to anti–PD-1 therapy.
IMPLICATIONS FOR PATIENT CARE: A potential application of PD-L1 PET is noninvasive, serial, comprehensive evaluation of PD-L1 expression not captured by single-site biopsies. This will enable better patient selection and improved prediction of outcomes to anti–PD-1 therapy.
Footnotes
Published online Mar. 21, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 9, 2023.
- Revision received February 13, 2024.