Visual Abstract
Abstract
In metastatic castration-resistant prostate cancer (mCRPC) patients treated with prostate-specific membrane antigen (PSMA)–targeted radioligand therapy (RLT), the recently proposed criteria for evaluating response to PSMA PET (RECIP 1.0) based on 68Ga- and 18F-labeled PET agents provided prognostic information in addition to changes in prostate-specific antigen (PSA) levels. Our aim was to evaluate the prognostic performance of this framework for overall survival (OS) in patients undergoing RLT and imaged with [18F]PSMA-1007 PET/CT and compare the prognostic performance with the PSA-based response assessment. Methods: In total, 73 patients with mCRPC who were scanned with [18F]PSMA-1007 PET/CT before and after 2 cycles of RLT were retrospectively analyzed. We calculated the changes in serum PSA levels (ΔPSA) and quantitative PET parameters for the whole-body tumor burden (SUVmean, SUVmax, PSMA tumor volume, and total lesion PSMA). Men were also classified following the Prostate Cancer Working Group 3 (PCWG3) criteria for ΔPSA and RECIP 1.0 for PET imaging response. We performed univariable Cox regression analysis, followed by multivariable and Kaplan–Meier analyses. Results: Median OS was 15 mo with a median follow-up time of 14 mo. Univariable Cox regression analysis provided significant associations with OS for ΔPSA (per percentage, hazard ratio [HR], 1.004; 95% CI, 1.002–1.007; P < 0.001) and PSMA tumor volume (per unit, HR, 1.003; 95% CI, 1.000–1.005; P = 0.03). Multivariable Cox regression analysis confirmed ΔPSA (per percentage, HR, 1.004; 95% CI, 1.001–1.006; P = 0.006) as an independent prognosticator for OS. Kaplan–Meier analyses provided significant segregation between individuals with versus those without any PSA response (19 mo vs. 14 mo; HR, 2.00; 95% CI, 0.95–4.18; P = 0.04). Differentiation between patients with or without progressive disease (PD) was also feasible when applying PSA-based PCWG3 (19 mo vs. 9 mo for non-PD and PD, respectively; HR, 2.29; 95% CI, 1.03–5.09; P = 0.01) but slightly failed when applying RECIP 1.0 (P = 0.08). A combination of both response systems (PCWG3 and RECIP 1.0), however, yielded the best discrimination between individuals without versus those with PD (19 mo vs. 8 mo; HR, 2.78; 95% CI, 1.32–5.86; P = 0.002). Conclusion: In patients with mCRPC treated with RLT and imaged with [18F]PSMA-1007, frameworks integrating both the biochemical (PCWG3) and PET-based response (RECIP 1.0) may best assist in identifying subjects prone to disease progression.
In patients with prostate cancer, treatment monitoring is essential but may be complex in individuals with high disease burden who are scheduled for varying therapies, including the recently approved lutetium vipivotide tetraxetan (Pluvicto; Novartis) (1,2). Despite favorable results in a randomized trial (3), a substantial portion of men will not benefit from treatment with [177Lu]Lu-labeled prostate-specific membrane antigen (PSMA) ligands (3,4). Thus, refined outcome assessment by an early identification of patients experiencing progressive disease (PD) is intensively sought. In this regard, monitoring the prostate-specific antigen (PSA) levels of patients undergoing treatment may allow the determination of high-risk individuals when applying a framework system such as criteria suggested by the Prostate Cancer Working Group 3 (PCWG3) (5). Changes in PSA levels, however, may have limited value in a castration-resistant setting for segregating between PD and true responses (6). Beyond biochemical evaluations, quantification of PSMA expression (as measured by SUVs) or PSMA-avid whole-body tumor volume may also assist in identifying patients experiencing PD under radioligand therapy (RLT) (7). Such conventional assessments exclusively focusing on quantified radiotracer accumulation, however, may ignore new lesions appearing on interim scans. To overcome this dilemma, the recently proposed response evaluation criteria in PSMA PET/CT (RECIP 1.0) were introduced, which were validated in a bicentric cohort of 124 patients staged with [68Ga]Ga-PSMA-11 and [18F]rhPSMA-7/7.3 (8). Interestingly, the authors concluded that a composite endpoint of PSA response and RECIP 1.0 was superior to PSA measurement only (8). A comparison among PSMA PET/CT-based frameworks for monitoring response to treatment, for example, PSMA PET progression criteria (9), adapted PERCIST (10,11), RECIST 1.1 (12), or RECIP 1.0 (8), has shown that the latter system appears to be the most promising (11). Whether RECIP 1.0 shows comparable results in patients exclusively imaged with [18F]PSMA-1007 PSMA PET has not yet been validated. Such findings, however, would be of relevance, as the latter radiotracer is increasingly used in the clinic because of a longer half-life and higher image resolution than for 68Ga-labeled PET agents (13,14).
Thus, in the present study, we aimed to evaluate the prognostic performance of RECIP 1.0 for overall survival (OS) in patients scheduled for RLT and imaged with [18F]PSMA-1007 PET/CT. Moreover, we also determined the associations of changes in PSA values, quantitative changes derived from [18F]PSMA-1007 PET, and biochemical and imaging response systems (PCWG3 and RECIP 1.0) with OS.
MATERIALS AND METHODS
Patient Cohort
In this single-center study, 172 patients with metastatic castration-resistant prostate cancer (mCRPC) who were treated with [177Lu]Lu-PSMA I&T at the University Hospital of Würzburg were retrospectively evaluated. Of these, 73 (42%) were eligible for inclusion in this study because they had previously undergone [18F]PSMA-1007 PET/CT and had an interim scan after 2 cycles of RLT, and survival data were also available. Eligibility criteria for RLT were as follows: prior therapy with enzalutamide/abiraterone and chemotherapy or refusal of or deemed unfit for chemotherapy, an Eastern Cooperative Oncology Group performance status no greater than 2, and PSMA-positive disease as confirmed on PSMA PET. Written informed consent was obtained from all subjects. The local ethics committee waived the requirement for further approval because of the retrospective nature of the study (waiver number 20220502 01). Parts of this cohort have already been reported previously (15–20). However, the analysis of PSMA PET/CT imaging before and after 2 cycles of RLT for association with OS in the context of RECIP 1.0 was not previously included.
Therapy Work-up
Before the initiation of RLT and 6 wk after the second cycle of RLT, all patients underwent a whole-body [18F]PSMA-1007 PET scan with CT for attenuation correction and anatomic coregistration. A detailed description of radiotracer synthesis and imaging procedures can be found in a previously published work (21).
PSA levels were measured at baseline and at follow-up. In addition, blood samples were collected at each cycle for serum chemistry (PSA level, creatinine, lactate dehydrogenase, aspartate aminotransferase, alkaline phosphatase, and C-reactive protein) and routine hematology (leukocytes, hemoglobin, and platelets). Samples were analyzed using an automated hematology analyzer (XN-9000; Sysmex) and a fully automated serum chemistry analyzer (Cobas; Roche Diagnostics) (19). Patient histories were reviewed retrospectively from medical records. For biochemical response, modified PCWG3 criteria were applied as provided in Table 1 (5).
Image Analysis
PET/CT images were analyzed with Syngo.via software (VB60 datasheet; Siemens Healthineers) for visual interpretation and the Beth Israel plug-in for FIJI (ImageJ) for segmentation (22). SUVmax, SUVpeak, and SUVmean of all lesions, as well as the total-body PSMA-avid tumor volume (PSMA-TV, in mL), were determined using a semiautomatic algorithm with a fixed threshold SUV of 3. All lesions were then reviewed by 1 expert reader, with inconclusive cases undergoing a second review by another expert reader. PSMA-TV was multiplied by SUVmean to calculate the total-lesion PSMA (TL-PSMA) (7). Differences between baseline and follow-up were calculated as the percentage increase or decrease for all quantitative PET parameters and PSA levels. The RECIP 1.0 classification was used to define the treatment response (Table 1) (8).
Treatment Protocol
[177Lu]Lu-PSMA I&T was synthesized as described previously (16). Every 6–8 wk, for a maximum of 8 cycles, patients received approximately 6.0 GBq of [177Lu]Lu-PSMA I&T. Patients with renal impairment had an approximately 20% reduction in activity (20).
Statistical Analysis
GraphPad Prism 9.3.0 was used for statistical analysis. Data are presented as median and range. OS was defined as the time from interim PSMA PET/CT to death (presented as median). Patients were considered to have PD in the combined assessment if they had PD according to either RECIP 1.0 or PCWG3 criteria or both. Univariable and multivariable Cox regression analyses were used to test for association with OS. Kaplan–Meier curves and log-rank tests were also performed. The hazard ratio (HR) and 95% CI are presented. The Harrell C statistic was used to compare Cox regression models for OS with higher Harrell C values, indicating a better model fit (23). Statistical significance was defined as a P value of less than 0.05.
RESULTS
Patient Characteristics
We included 73 patients with a median initial Gleason score of 9 (range, 6–10) and a median age of 72 y (range, 46–88 y). Subjects were treated with a median of 4 cycles of [177Lu]Lu-PSMA I&T (cumulative activity, 19.1 GBq; range, 11.2–50.9 GBq). Median OS was 15 mo, and 31 patients had died at follow-up. The median follow-up was 14 mo.
The median baseline PSA value was 107 ng/mL (range, 2.9–3,590 ng/mL). Before initiation of RLT, nearly all patients (72/73, 98.6%) presented with bone metastases, 49 of 73 patients (67.1%) showed lymph node metastases, and 28 of 73 patients (38.4%) showed visceral metastases (21.9% liver and 15.1% lung). Prior systemic treatments included antihormonal agents (73/73, 100%), enzalutamide (58/73, 79.5%), abiraterone (50/73, 68.5%), and chemotherapy (58/73, 79.5%).
PET quantification yielded the following median baseline values: PSMA-TV, 730.7 cm3 (range, 5.0–3,301 cm3); TL-PSMA, 5,135 cm3 (range, 20.2–33,441 cm3); SUVmax, 39.7 (range, 6.6–190.9); SUVpeak, 19.3 (range, 4.3–94.1), and SUVmean, 7.6 (range, 4.0–16.3) (Table 2).
Patient Characteristics
Changes of PET-Based Parameters and PSA After 2 Treatment Cycles
After 2 cycles of RLT with [177Lu]Lu-PSMA I&T, 30 of 73 (41.1%), 37 of 73 (50.7%), 61 of 73 (83.6%), 58 of 73 (79.5%), and 62 of 73 (84.9%) patients showed a reduction of PSMA-TV, TL-PSMA, SUVmax, SUVpeak, and SUVmean, respectively. Any reduction of PSA levels was found in 49.3% of patients. Table 3 provides an overview of respective changes.
Change After 2 Cycles of RLT
According to RECIP 1.0, 8 of 73 patients (11%) were classified as partial response, 36 of 73 patients (49.3%) were classified as stable disease (StD), and 29 of 73 patients (39.7%) were classified as PD. According to the PCWG3 criteria, 22 of 73 patients (30.1%) were classified as partial response, 18 of 73 patients (24.7%) were classified as StD, and 33 of 73 patients (45.2%) were classified as PD.
Changes in Tumor Volume Are Associated with OS
Univariable Cox regression analysis provided significant associations with OS only for changes in serum PSA values (per percentage, HR, 1.004; 95% CI, 1.002–1.007; P = 0.001) and changes in PSMA-TV (per unit, HR, 1.003; 95% CI, 1.000–1.005; P = 0.03). Any PSA decrease (HR, 0.50; 95% CI, 0.23–1.09; P = 0.08) and a PSA decrease of greater than 50% (HR, 0.43; 95% CI, 0.17–0.97; P = 0.05) slightly failed to reach significance. The change in TL-PSMA showed no relevant link to OS, and the appearance of new lesions (P ≥ 0.24) was also not significantly associated with shorter survival (P ≥ 0.10).
In a multivariable Cox regression analysis with an adjustment for a change in PSMA-TV and the appearance of new lesions, the change in serum PSA levels (per percentage, HR, 1.004; 95% CI, 1.000–1.006; P = 0.03) remained an independent variable for OS (Table 4).
Univariable and Multivariable Cox Regression
RECIP 1.0 and PCWG3 Can Identify Patients Experiencing Treatment Failure
Using RECIP 1.0 and PCWG3 classification in a Cox regression analysis, framework-defined PD was also associated with shorter OS for PCWG3 PD (HR, 2.3; 95% CI, 1.02–5.1; P = 0.04) or PD on the basis of PCWG3 and RECIP 1.0 (HR, 3.3; 95% CI, 1.5–7.8; P = 0.004). RECIP 1.0 PD (HR, 2.1; 95% CI, 0.96–4.6; P = 0.06), however, failed to reach significance. The combination of PCWG3 and RECIP 1.0 also showed the highest C-index of 0.69 (0.60 for RECIP 1.0 and 0.63 for PCWG3; Table 4).
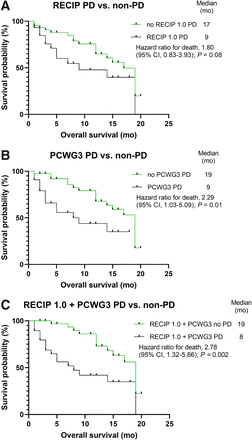
Kaplan–Meier analyses confirmed those findings by providing significant segregation between individuals without versus those with PCWG3 PD (19 mo vs. 9 mo; HR, 2.29; 95% CI, 1.03–5.09; P = 0.01) and between individuals without versus those with combined (RECIP 1.0 and PCWG3) PD (19 mo vs. 8 mo; HR, 2.78; 95% CI, 1.32–5.86; P = 0.002; Fig. 1). RECIP 1.0 alone, however, could not significantly differentiate between individuals without versus those with PD (P = 0.08). Moreover, we observed significant survival differences in patients with versus those without a PSA response of greater than 50% (17 mo vs. 14 mo; HR, 2.17; 95% CI, 1.07–4.43; P = 0.04) and in patients with versus those without any PSA response (19 mo vs. 14 mo; HR, 2.00; 95% CI, 0.95–4.18; P = 0.04). The appearance of new lesions on PET failed to reach significance (P = 0.11; Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org).
Kaplan–Meier analyses of patients grouped according to RECIP 1.0, PCWG3 PSA, and combined assessment for PD or non-PD. Patients with PD according to RECIP 1.0 (OS, 9 mo vs. 17 mo) (A), PCWG3 PSA (OS, 9 mo vs. 19 mo) (B), and combined PD (OS, 8 mo vs. 19 mo) (C) showed shorter OS. However, only segregations based on PCWG3 and PCWG3 with RECIP 1.0 reached significance.
In 40 patients, who were classified as non-PD according to the PCWG3 criteria, RECIP 1.0 PD was recorded in 5 individuals (12.5%). These 5 subjects showed an OS that was significantly shorter than that of RECIP 1.0 non-PD patients (7 mo vs. 19 mo; HR, 3.06; 95% CI, 0.59–15.9; P < 0.02). In contrast, 44 subjects were classified as non-PD according to RECIP 1.0, but 9 patients (20.5%) would have been classified with PD following the PCWG3 criteria. The survival difference was even more pronounced using the PCWG3 criteria (3 mo vs. 19 mo for PD and non-PD, respectively; HR, 6.09; 95% CI, 0.86–43.0; P < 0.001; Supplemental Fig. 2).
DISCUSSION
When the frameworks for PSA (PCWG3) and PSMA PET response (RECIP 1.0) were applied, patients with PCWG3 PD had an OS that was significantly shorter than that of non-PD patients, whereas this was not significant for RECIP 1.0. The combination of both classifications, however, provided the most accurate evaluation of identifying patients not responding to RLT.
Comparable to previous findings in a mixed cohort imaged with 68Ga- and 18F-labeled PSMA PET (8), the present study indicates that established biochemical and PET response systems are also applicable to a cohort of men imaged exclusively with 18F-labeled agent PSMA-1007.
In approximately 50% of our patients, we observed a PSA reduction after 2 cycles of PSMA RLT, which was significantly associated with improved OS. This confirms the results of a previous metaanalysis (24) and highlights the overall importance of PSA response in patients undergoing PSMA RLT. Furthermore, blood collection was performed after 2 cycles (i.e., 13–14 wk after initiation of RLT), which is in line with the recommended timing of the PCWG3 criteria (5). Interestingly, any PSA response and a PSA reduction of at least 50% (indicative for response according to the PCWG3 criteria (5)) yielded comparable results for survival.
Beyond biochemical investigations, we also analyzed the percentage changes of TL-PSMA and PSMA-TV, and only the latter metric showed a significant association with OS in a univariable analysis but missed significance in a multivariable model. This is in contrast to findings published by Grubmüller et al., whose evaluation was not conducted after 2 cycles of RLT (as in the present study) but at the end of therapy (25). Rosar et al. used a classification with a cutoff of +30% and −30% for PSMA-TV or TL-PSMA to define PD and partial response, whereas StD was defined as changes between those cutoffs. Using that classification, they reported that patients with StD or PD based on TL-PSMA had a higher risk of death (HR, 2.76), whereas PSA response according to the PCWG3 criteria did not reach significance (26). Those discrepant findings relative to our results, however, may be partially explained by the different radiotracers used for imaging ([68Ga]Ga-PSMA-11) and treatment ([177Lu]Lu-PSMA-617) when compared with the current study ([18F]PSMA-1007 and [177Lu]Lu-PSMA I&T). As such, future investigations may also determine the prognostic value of PET-based quantification under RLT but focusing on other also clinically relevant theranostic pairs, for example, 18F-piflufolastat (Pylarify; Lantheus) combined with [177Lu]Lu-PSMA-617. Moreover, the intensity of PSMA expression may also be crucial in the context of OS prognostication (27). For instance, in [177Lu]Lu-PSMA-617–treated individuals, only PSMA PET with a certain level of target expression may be suited for outcome prediction when PSMA-TV reduction is considered (28). Of note, in our study, respective quantitative parameters such as SUVmean or SUVpeak were still substantially elevated.
In the cohort used to establish RECIP 1.0 response, the best cutoff for progression was defined as a 20% increase of PSMA-TV, whereas the most appropriate threshold for response was set by a 30% reduction (using [68Ga]Ga-PSMA-11 and [18F]rhPSMA-7/7.3). OS was improved in patients with RECIP 1.0 partial response and StD compared with OS in patients with RECIP 1.0 PD (8). Those results were confirmed in a mixed cohort of [68Ga]Ga-PSMA-11 and [18F]PSMA-1007, with patients with PD having a significantly shorter OS than patients without PD (HR, 2.69) (29). In our cohort only including patients imaged with [18F]PSMA-1007 PET, we cannot fully confirm the prognostic ability of RECIP 1.0 as survival was prolonged in patients with non-PD but without reaching significance relative to that with RECIP 1.0 PD. Hence, ours and previous findings (8,29) indicate that this framework may be useful for identifying subjects prone to PD, but reevaluation may be required, for example, if PSMA PET radiotracers other than those discussed herein are used.
In patients with PCWG3-based non-PD, almost 13% of the patients had RECIP 1.0 PD with an OS inferior to that of patients without PD, which is virtually identical to the 14% reported by Gafita et al. (8). Interestingly, in our cohort, 4 of these 5 patients showed reduction in PSA values but not by 50% or greater. In contrast, approximately 20% of patients with RECIP 1.0–classified non-PD were classified with PCWG3 PD and also showed inferior OS. As such, those discordant assignments to the PD versus non-PD groups based on PCWG3 and RECIP 1.0 along with outcome differences emphasize that one system alone may not be sufficient to identify high-risk patients prone to therapeutic failure. In this regard, a previous study has also shown that the prognostic ability for PD as defined by PSA is slightly inferior to that by RECIP 1.0 or the combination of PSA and RECIP 1.0 (8). In our cohort, the prognostic ability for PD as defined by RECIP 1.0 was inferior to that with PCWG3 criteria and, in particular, with a combination of PCWG3 and RECIP 1.0, which achieved the best prognostic value (C-index, 0.69). Thus, our results may favor a more widespread adoption of combined PSA and PSMA evaluations (PCWG3 and RECIP 1.0) in the clinic or in future trials.
It is noteworthy that the RECIP 1.0 definitions use the appearance of new lesions to define progression. Occurrence of new metastases, however, does not necessarily indicate PD if tumor volume is stable, and this criterion has been incorporated in this framework to avoid overstaging (8). Of note, this approach is also supported by our findings, as new metastases on PET alone were not associated with shorter OS.
Limitations include the retrospective character of this study and the small number of patients in certain subcategories, for example, between patients with new lesions versus no new lesions or between PSA-based responders and nonresponders. However, our findings indicate that for [18F]PSMA-1007, RECIP 1.0 response alone should be used with caution. In this regard, a combination of PSA and molecular imaging response classifications is most suitable to identify subjects prone to treatment failure. Nonetheless, quantitative assessments are time-consuming, and thus, our investigation may be repeated using the recently proposed visual RECIP 1.0 (30).
CONCLUSION
In men undergoing PSMA RLT and imaged with [18F]PSMA-1007 PET, changes in PSA levels, but not PSMA-TV, emerged as an independent prognosticator for OS. In the context of [18F]PSMA-1007, treatment evaluation may rather not be conducted based only on RECIP 1.0. A dual-response assessment using criteria from PCWG3 and RECIP 1.0, however, provided the best prognostic ability to identify patients with mCRPC at risk of PD. Taken together, ours and previous findings highlight the clinical relevance of structured biochemical and imaging response assessments in patients scheduled for RLT.
DISCLOSURE
Andreas Buck and Kerstin Michalski have received speaker honoraria from Novartis. Rudolf Werner has received speaker honoraria from Novartis and reports advisory board work for Novartis/AAA and Bayer. This work was supported by the IZKF Wuerzburg (grant Z-2/91 to Wiebke Schlötelburg) and the Bavarian Cancer Research Center (personal grant to Kerstin Michalski). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can biochemical (changes in PSA levels according to PCWG3) and image-based response criteria (applying RECIP 1.0) predict OS in prostate cancer patients treated with [177Lu]Lu-PSMA I&T and imaged with [18F]PSMA-1007 PET?
PERTINENT FINDINGS: PCWG3 criteria were able to distinguish PD versus non-PD individuals, but the combination of PCWG3 and RECIP provided the strongest prognostic value.
IMPLICATIONS FOR PATIENT CARE: In patients with prostate cancer treated with [177Lu]Lu-PSMA I&T, the combination of PCWG3 and RECIP 1.0 (from [18F]PSMA-1007 PET) can identify subjects prone to PD, thereby emphasizing the importance of biochemical and image-based standardized response assessment.
Footnotes
Published online Mar. 7, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication September 19, 2023.
- Revision received January 5, 2024.