Visual Abstract
Abstract
This study evaluated the potential to reduce the scan duration in dopamine transporter (DAT) SPECT when using a second-generation multiple-pinhole (MPH) collimator designed for brain SPECT with improved count sensitivity and improved spatial resolution compared with parallel-hole and fanbeam collimators. Methods: The retrospective study included 640 consecutive clinical DAT SPECT studies that had been acquired in list mode with a triple-head SPECT system with MPH collimators and a 30-min net scan duration after injection of 181 ± 10 MBq of [123I]FP-CIT. Raw data corresponding to scan durations of 20, 15, 12, 8, 6, and 4 min were obtained by restricting the events to a proportionally reduced time interval of the list-mode data for each projection angle. SPECT images were reconstructed iteratively with the same parameter settings irrespective of scan duration. The resulting 5,120 SPECT images were assessed for a neurodegeneration-typical reduction in striatal signal by visual assessment, conventional specific binding ratio analysis, and a deep convolutional neural network trained on 30-min scans. Results: Regarding visual interpretation, image quality was considered diagnostic for all 640 patients down to a 12-min scan duration. The proportion of discrepant visual interpretations between 30 and 12 min (1.2%) was not larger than the proportion of discrepant visual interpretations between 2 reading sessions of the same reader at a 30-min scan duration (1.5%). Agreement with the putamen specific binding ratio from the 30-min images was better than expected for 5% test–retest variability down to a 10-min scan duration. A relevant change in convolutional neural network–based automatic classification was observed at a 6-min scan duration or less. Conclusion: The triple-head SPECT system with MPH collimators allows reliable DAT SPECT after administration of about 180 MBq of [123I]FP-CIT with a 12-min scan duration.
SPECT of striatal dopamine transporter (DAT) availability with N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-123I-iodophenyl)nortropane ([123I]FP-CIT) is widely used to support the diagnostic work-up in patients with a clinically uncertain parkinsonian syndrome or suspicion of dementia with Lewy bodies (1–6).
Multiple-pinhole (MPH) collimator technology has the potential to concurrently improve spatial resolution and count sensitivity compared with imaging with conventional parallel-hole and fanbeam collimators in clinical SPECT of small organs (7), including DAT SPECT with [123I]FP-CIT (8–15).
A recent prospective study showed that MPH collimators improve intra- and interreader agreement and the certainty of the visual interpretation of DAT SPECT compared with low-energy, high-resolution, high-sensitivity collimators (16). A technical performance evaluation of the triple-head camera with MPH collimators used in this previous (and in the current) study found a peak system sensitivity of 675 cps/MBq, which is about 3 times higher than the typical system sensitivity of double-head cameras with conventional parallel-hole or fanbeam collimators (7,15). This finding suggests that the triple-head camera equipped with MPH collimators allows a considerable reduction in scan duration in DAT SPECT. This reduction is desirable for better patient comfort, reduced risk of motion artifacts, and reduced costs (in terms of camera occupancy).
Against this background, the current study evaluated the impact of scan duration on MPH DAT SPECT with respect to visual interpretation, conventional semiquantitative analysis, and automatic classification with a deep convolutional neural network. The study retrospectively included 640 MPH DAT SPECT studies from clinical routine that had been acquired in list mode and therefore allowed realistic simulation of reduced scan duration.
MATERIALS AND METHODS
Patients
MPH DAT SPECT with [123I]FP-CIT had been performed on 665 consecutive patients with a clinically uncertain parkinsonian syndrome or suspected dementia with Lewy bodies. Thirteen patients were excluded because the [123I]FP-CIT dose was less than 150 MBq. Twelve patients were excluded because of relevant structural or vascular lesions in the striata or midbrain on MRI. The remaining 640 patients were included (age, 67.2 ± 11.4 y; range, 26–91 y; 44.2% women). To guarantee that the included patient sample was representative of the clinical routine at our site, no further eligibility criteria were applied.
A waiver of informed consent for the retrospective analysis of the anonymized data was obtained from the ethics review board of the general medical council of the state of Hamburg, Germany.
SPECT Imaging
The SPECT acquisition started 203 ± 25 min (range, 135–335 min) after intravenous injection of 181 ± 10 MBq (range, 156–215 MBq) of [123I]FP-CIT. In total, 90 projection views (30 per head, 120° scan arc) at angular steps of 4° were acquired in list mode with an AnyScan Trio (Mediso) triple-head, general-purpose camera equipped with second-generation, general-purpose brain MPH collimators designed for high count sensitivity at the center of the field of view with a rather broad peak of the sensitivity profile for improved stability with respect to off-center positioning (16). A detailed description of the MPH collimator is given in the supplemental materials (“Multiple-Pinhole Collimator”; Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org) (15,17,18).
The acquisition time per projection was 60 s, resulting in a 30-min total net scan duration. The energy window was set to 143–175 keV. The distance between the center-of-rotation axis and the pinhole focal plane was fixed to 140 mm. A helical acquisition mode with a 40-mm total table displacement was used to avoid axial undersampling (19,20).
Projection data corresponding to reduced scan durations of 20, 15, 12, 8, 6, and 4 min were obtained by restricting the events to a proportionally reduced time interval of the list-mode data for each projection angle. For each projection, the earliest sequential decay events were selected (rather than random selection from all events). Projection data were sorted into 256 × 256 matrices with a 2.13 × 2.13 mm pixel size, separately for each scan duration.
Transaxial images of 128 × 128 cubic pixels with a 1.8-mm edge length were reconstructed with the Monte Carlo photon simulation engine and iterative 1-step-late maximum-a-posteriori expectation maximization implemented in the camera software (24 iterations, 2 subsets) (15,21). Attenuation and scatter correction do not affect the ability of DAT SPECT to discriminate between a normal striatal signal and a neurodegeneration-typical reduction (22). Hence, correction for photon attenuation and scatter was not performed to avoid variability of no interest between images of the same patient with different scan durations, as might be caused by variability in the outer contour of the head for postreconstruction attenuation and scatter correction.
Image Preprocessing
Individual DAT SPECT images were stereotactically normalized (affine) to the anatomic space of the Montreal Neurologic Institute using the Normalize tool of the Statistical Parametric Mapping software package (version SPM12) and a set of custom DAT SPECT templates representative of normal and different levels of a neurodegeneration-typical reduction in striatal uptake as the target (23). For each of the 640 patients, stereotactic normalization was performed first for the SPECT image from the full 30-min projection data. The images corresponding to shorter scan durations from the same raw data were stereotactically normalized using the same transformation as for the 30-min images.
Intensity was normalized by voxelwise scaling to the individual 75th percentile of the voxel intensity in a reference region comprising the whole brain without striata, thalamus, medial temporal lobe, brain stem, cerebellum, and ventricles (24). The reference value for intensity scaling was obtained separately for each scan duration. The scaled images are semiquantitative images representing the distribution volume ratio.
Visual Interpretation
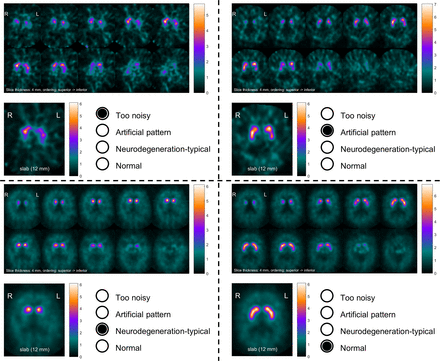
A standardized display (Fig. 1) was used for visual interpretation of the DAT SPECT distribution-volume-ratio images, similar to the display used in clinical routine at our site. Visual interpretation was performed by a reader with about 20 y of experience in clinical DAT SPECT reading (≥3,000 cases). The reader was unaware of any clinical data and was asked to use a 3-step approach to visual interpretation. In the first step, the reader decided whether statistical image quality was adequate for visual interpretation or whether the image was too noisy (Fig. 1). If statistical image quality was adequate, the reader decided in the second step whether there was an artificial reduction in striatal [123I]FP-CIT uptake such as a more prominent reduction in the caudate nucleus than in the putamen or a barbell-shaped appearance of the striatum (Fig. 1), the rationale being that artificial reduction most likely indicated an artifact, because patients with relevant lesions on MRI had been excluded. If there was no artificial reduction, the reader was asked to categorize the case as showing a neurodegeneration-typical reduction or normal striatal [123I]FP-CIT uptake (Fig. 1).
Standardized display for visual interpretation of 4 cases: 4-min scan considered too noisy for visual interpretation (upper left), 4-min scan interpreted as artificial because of barbell shape of left striatum (upper right), and two 30-min scans interpreted as neurodegeneration-typical reduction (lower left) and normal (lower right). Top of each panel shows 10 transversal distribution-volume-ratio slices of 4-mm thickness from superior to inferior edge of striatum with maximum of color table individually scaled to maximum intensity in 10 images. Bottom of each panel shows transversal distribution-volume-ratio image of 12-mm thickness through center of striatum with maximum of color table scaled to fixed upper distribution-volume-ratio threshold optimized previously.
The 5,120 cases (640 patients × 8 scan durations) were presented in randomized order in a 5,120-page Portable Document Format file with 1 case per page. Visual interpretation was performed by clicking 1 of the 4 buttons in the display (Fig. 1). To assess intrareader variability (as a benchmark for variability between different scan durations), all 5,120 cases were interpreted a second time by the same reader using a second 5,120-page Portable Document Format file with the cases randomized differently. The time between the 2 reading sessions was 2 wk.
Cases whose interpretation was discrepant between the 2 reading sessions were read a third time by the same reader to obtain an intrareader consensus.
Semiquantitative Analysis
A detailed description of the semiquantitative analysis is given in the supplemental materials (“Specific Binding Ratio Analysis” (23–30)). In brief, the unilateral [123I]FP-CIT specific binding ratio (SBR) in the left and right putamina was obtained by hottest-voxels analysis of the stereotactically normalized distribution-volume-ratio image using large unilateral putamen masks predefined in the space of the Montreal Neurologic Institute as described previously (30).
Automatic Classification by Deep Convolutional Neural Network
A randomly selected subset of 427 (=2/3) of the DAT SPECT scans with a 30-min duration and visual interpretation by the experienced reader (intrareader consensus) was used to train a convolutional neural network for automatic binary classification of DAT SPECT scans. Details are given in the supplemental materials (“Convolutional Neural Network”; Supplemental Fig. 2 (31–33)).
The performance of the network was first tested in the remaining 213 DAT SPECT scans with a 30-min duration. To assess the impact of scan duration on the network’s performance, the same network was applied to the same 213 test cases but with a reduced scan duration. No attempt was made to optimize the network for scan durations of less than 30 min.
Semiquantitative analyses and network-based classification included all cases; neither too-noisy nor artificial cases (according to visual interpretation) were excluded.
Statistical Analysis
The Cochran Q test for related samples was used to test for an impact of scan duration on the proportion of too-noisy cases according to visual inspection in the whole dataset, on the proportion of artificial cases among cases with adequate statistical image quality, on the proportion of cases with discrepant visual interpretation between the 2 reading sessions, and on the proportion of cases with a discrepant intrareader consensus at a reduced scan duration compared with the intrareader consensus on the 30-min image. If the Cochran Q test demonstrated a significant effect of scan duration (P < 0.05), pairwise comparisons of scan durations were performed with Bonferroni adjustment for multiple tests (28 pairs from 8 different scan durations). IBM SPSS (version 27) was used for these analyses.
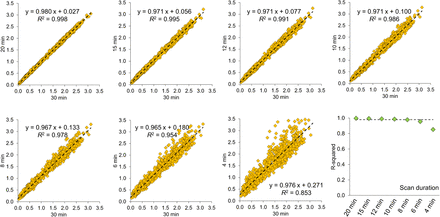
The impact of scan duration on the unilateral putamen SBR (n = 1,280) was assessed by scatterplots and regression analysis to determine the coefficient of determination (R2) of the SBR from the full 30-min scan by the SBR from a reduced scan duration. The threshold on R2 for the impact of a reduced scan duration on the SBR to be relevant was fixed at 0.98, which would be expected alone because of the 5% variability in putamen SBR in short-term test–retest DAT SPECT of the same patient (“Relevant Loss of SBR Determination” in the supplemental materials; Supplemental Fig. 3 (34)). Thus, the impact of a reduced scan duration was considered relevant if R2 was below 0.98.
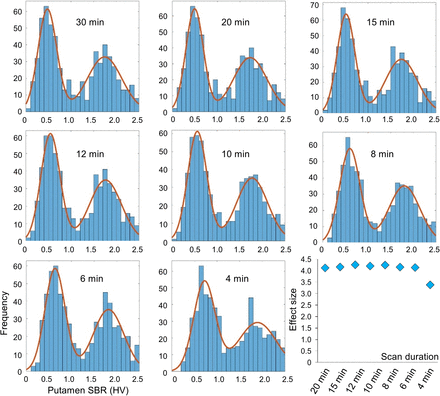
The impact of scan duration on the discriminative power of the putamen SBR was tested as described previously (22). In brief, the distribution of the putamen SBR (minimum of both hemispheres, n = 640) was characterized by a histogram with a 0.1 bin width. The resulting histogram was fitted by the sum of 2 gaussians: Eq. 1where A1 and A2 are the amplitudes, M1 and M2 are the mean values, and SD1 and SD2 are the SD of the gaussian functions. The MATLAB routine fminsearch with default parameter settings was used for this purpose.
Eq. 1where A1 and A2 are the amplitudes, M1 and M2 are the mean values, and SD1 and SD2 are the SD of the gaussian functions. The MATLAB routine fminsearch with default parameter settings was used for this purpose.
The power of the SBR to differentiate between normal and reduced DAT SPECT was estimated by the effect size d of the distance between the 2 gaussians computed as the differences between the mean values scaled to the pooled SD: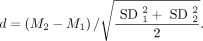 Eq. 2
Eq. 2
The cutoff c for differentiation between normal and reduced SBRs was selected halfway between M1 and M2 in units of SD, that is Eq. 3
Eq. 3
The histogram analysis was performed separately for each scan duration.
RESULTS
None of the DAT SPECT images were considered too noisy for visual interpretation down to an 8-min scan duration (Fig. 2A). The proportion of too-noisy images was 0.3% at a 6-min scan duration and 3.0% at a 4-min scan duration. The impact of scan duration on the proportion of too-noisy images was highly significant (P < 0.0005). Pairwise testing showed that the proportion of too-noisy images was significantly higher at 4 min than at any of the longer scan durations (all P < 0.0005). The difference between the 6-min scan duration and the longer scan durations was not significant (all P = 1.000).
(A) Results of visual interpretation. (B) Transversal 12-mm SPECT images with 4-min vs. 30-min scan durations for 4 representative cases: too noisy on 4-min scan (male, 83 y, 175 MBq, 210-min uptake period), artificial on 4-min scan (male, 81 y, 182 MBq, 188-min uptake period), intrareader discrepancy (normal vs. reduced) on 4-min scan (male, 68 y, 183 MBq, 177-min uptake period), and discrepant classification between 4-min (reduced) and 30-min (normal) scans (female, 77 y, 196 MBq, 268-min uptake period).
Among the 621 DAT SPECT images with sufficient statistical quality at all scan durations, the proportion with an artificial pattern was no more than 0.2% for any scan duration down to 8 min (Fig. 2A). It was 1.3% at a 6-min scan duration and 6.4% at a 4-min scan duration. The impact of scan duration on the proportion of artificial images was highly significant (P < 0.0005). Pairwise testing showed that the proportion of artificial images was significantly higher at 4 min than at any of the longer scan durations (all P < 0.0005). The difference between the 6-min scan duration and the longer scan durations was not significant (all P ≥ 0.575).
Among the 581 DAT SPECT images that were neither too noisy nor artificial at any scan duration, the proportion of cases with discrepant visual categorization between the 2 reading sessions ranged between 1.0% and 1.9%, without a significant effect of scan duration (P = 0.822, Fig. 2A).
The proportion of cases with a discrepant visual intrareader consensus at a reduced scan time compared with the intrareader consensus on the 30-min image among these 581 DAT SPECT images ranged between 0.7% and 1.2% for scan durations from 20 to 8 min (Fig. 2A). The proportion increased to 1.9% and 3.3% at 6- and 4-min scan durations, respectively. The impact of scan duration on the rate of discrepant consensus interpretation was highly significant (P < 0.0005). Pairwise testing showed that the proportion of discrepant interpretations compared with the 30-min image was significantly higher at 4 min than at 8 min or more (all P < 0.0005). The proportion of discrepant cases at a 6-min scan duration did not differ from the proportion at a 4-min scan duration (P = 0.093) or longer (P ≥ 0.268).
Representative examples of too-noisy, artificial, intrareader-discrepant, and between-scan-duration–discrepant cases are shown in Figure 2B. Retrospective inspection of the cases with a concordant visual interpretation across all scan durations revealed only minor differences in the visual appearance of the SPECT images down to a 4-min scan duration in most cases (Fig. 3).
Representative examples in which scan duration had no relevant impact down to 4 min (transversal 12-mm images; top: female, 44 y, 200 MBq, 186-min uptake period; bottom: female, 72 y, 200 MBq, 220-min uptake period).
Scatterplots of the putamen SBR with a reduced scan duration versus a 30-min scan duration are shown in Figure 4. R2 decreased with reduced scan durations from 0.998 at a 20-min duration to 0.853 at a 4-min duration. It dropped below the threshold of 0.98 for clinical relevance at an 8-min duration.
Scatterplots of unilateral putamen SBR (n = 1,280) with reduced vs. 30-min scan duration, and plot of R2. Dashed lines indicate R2 expected for putamen SBR from 2 short-term repeat DAT SPECT scans of same patient with 5% test–retest variability.
The histograms of the putamen SBR at different scan durations and their fit by the sum of 2 gaussians are shown in Figure 5. The parameters obtained by the fit are given in Supplemental Table 1. The effect size d of the distance between the 2 gaussians was stable from a 30-min to a 6-min scan duration and then dropped by about 20% at a 4-min scan duration.
Histograms of putamen SBR (minimum of both hemispheres, n = 640), and effect size d of distance between 2 gaussian functions (according to Eq. 2). Fit by sum of 2 gaussians is indicated by continuous line. Remaining fit parameters are given in Supplemental Table 1. HV = hottest-voxels.
The results of the automatic network-based classification are summarized in Figure 6. A relevant loss of accuracy was observed for scan durations of 6 min or less.
Balanced accuracy of network trained for automatic classification of MPH DAT SPECT with 30-min scan duration in same 213 test cases with varying scan duration.
DISCUSSION
The primary finding of this study is that the triple-head SPECT camera equipped with MPH collimators allows reliable DAT SPECT after administration of the standard dose of about 180 MBq of [123I]FP-CIT with a 12-min scan duration, independent of the interpretation method (visual, conventional semiquantitative analysis, or deep learning–based automatic classification). This scan duration represents a reduction of at least 50% compared with the typical 25–40 min according to the European Association of Nuclear Medicine/Society of Nuclear Medicine practice guideline for DAT SPECT when using a double-head camera with conventional collimators (5).
Regarding visual interpretation, none of the 640 DAT SPECT images was considered too noisy or artificial at a 12-min scan duration (Fig. 2). The proportion of cases with a discrepant visual interpretation between the 2 reading sessions of the same reader was not larger at a 12-min scan duration than at a 30-min scan duration (1.0% vs. 1.5%). The proportion of cases with a discrepant visual classification between a 12-min and a 30-min scan duration (1.2%) was similar to the proportion of intrareader-discrepant cases at 30 min (1.5%). Thus, the reduction of scan duration from 30 to 12 min had no impact on visual interpretation. Regarding semiquantitative analysis, a relevant impact was observed only at a scan duration of 8 min or less (Figs. 4 and 5). Automatic classification with the convolutional neural network showed a relevant impact only at a scan duration of 6 min or less (Fig. 6).
The current study provides strong evidence against an impact of reduced scan duration down to 12 min with MPH SPECT, given the rather large sample size (n = 640) and that no specific eligibility criteria were imposed. In response to this finding, the scan duration of clinical DAT SPECT with the triple-head SPECT camera equipped with MPH collimators and the standard dose of about 180 MBq of [123I]FP-CIT was reduced to 12 min at our site.
Further reduction of the scan duration to 6 min did not impact DAT SPECT image interpretation in most cases (≥95%, Figs. 2–6). Thus, a 12-min scan duration can be considered a safe choice. Under difficult conditions (e.g., patients with severe pain when lying on the examination table or agitated patients with dementia with Lewy bodies), an attempt at a 6-min duration might be made. If the resulting image quality appears adequate visually (neither too noisy nor artificial), the risk of misinterpretation is only marginally increased (Fig. 2). If the resulting image quality does not appear adequate, the scan might be repeated with a longer duration.
Reduction of scan duration with MPH collimators is based on their increased count sensitivity compared with conventional collimators (7–15,35). More precisely, the total number of striatal counts detected with the triple-head SPECT camera with MPH collimators with a 12-min scan duration is about the same as detected with a double-head SPECT camera with conventional parallel-hole or fanbeam collimators during a 25- to 30-min scan (16). Thus, novel software approaches such as deep learning–based image enhancement or denoising (36) might be added to further reduce the scan duration in DAT SPECT with MPH collimators.
Reduction of scan duration to 12 min or even lower might allow early-phase SPECT imaging after injection of [123I]FP-CIT to provide a surrogate image of regional cerebral blood flow (e.g., to discriminate between different neurodegenerative parkinsonian syndromes) as described for PET with [123I]FP-CIT (37). However, this possibility needs to be tested in future studies, since system sensitivity with MPH collimators decreases toward the edges of the field of view and, therefore, improvement in sensitivity is lower for cortical brain regions than for the striata (16).
A limitation of the current study is that the reduced scan duration was simulated by restricting the events used for image reconstruction to a proportionally reduced time interval of the list-mode data for each projection angle. As a consequence, the current study does not allow testing for potential improvement of diagnostic performance by less involuntary head motion during a reduced scan duration. Another limitation is that the visual interpretation was performed by only a single experienced reader. However, at the safe choice of a 12-min scan duration, close inspection of the images was required to see any effect of reduced scan duration at all (Fig. 3). There was no change in image quality at a 12-min scan duration that might affect interpretation by less experienced readers. This was further supported by imagewise correlation analyses described in the supplemental materials (“Imagewise Correlation”; Supplemental Fig. 4). Finally, images with reduced scan durations were stereotactically normalized using the transformation derived for the corresponding 30-min image. This might have caused overly optimistic performance estimates (the 30-min scan is not available in clinical practice with a reduced scan duration). Repeat analyses in which the affine transformation into the anatomic standard space was estimated from the image with a reduced scan duration itself (i.e., without reference to the 30-min images) resulted in an R2 (of the 30-min SBR by the 12-min SBR) that was slightly lower (0.98 vs. 0.99) but still compatible with the nonrelevance threshold. Thus, additional variability by independent stereotactic normalization did not have a relevant impact on the diagnostic performance of MPH DAT SPECT with a 12-min scan duration. The same might be assumed for attenuation and scatter correction, particularly when the delineation of the outer contour of the head (required for postreconstruction uniform attenuation and scatter correction) is based on the transformation used for stereotactic normalization (38).
CONCLUSION
The triple-head SPECT system with MPH collimators allows reliable DAT SPECT with a 12-min scan duration when a standard dose of about 180 MBq of [123I]FP-CIT is administered. The improved count sensitivity might also be used to reduce the radioactivity dose administered to patients.
DISCLOSURE
Balazs Szabo, Akos Kovacs, and Attila Forgacs are employees of Mediso Medical Imaging Systems, Budapest, Hungary. However, the nonemployee authors had full control of the data and information that might present a conflict of interest for the employee authors. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the minimum scan duration for DAT SPECT with a general-purpose triple-head camera equipped with brain-specific MPH collimators?
PERTINENT FINDINGS: The retrospective study included 640 MPH DAT SPECT images that had been acquired at a 30-min duration in list mode and therefore allowed realistic simulation of reduced scan duration. Reduction of scan duration to 12 min had no impact on the interpretation of the SPECT images independent of the interpretation method (visual, conventional semiquantitative analysis, or deep learning–based automatic classification).
IMPLICATIONS FOR PATIENT CARE: The triple-head SPECT system equipped with MPH collimators allows reliable DAT SPECT with a 12-min scan duration when a standard 180-MBq dose of [123I]FP-CIT is administered.
Footnotes
Published online Jan. 18, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication July 2, 2023.
- Accepted for publication November 28, 2023.