Visual Abstract
Abstract
Quantitative evaluation of prostate-specific membrane antigen (PSMA)–targeting PET/CT remains challenging but is urgently needed for the use of standardized PET-based response criteria, such as the PSMA PET/CT consensus statement or Response Evaluation Criteria in PSMA PET/CT (RECIP 1.0). A recent study evaluated the prognostic value of whole-body tumor volume using a semiautomatic method relying on a 50% threshold of lesion SUVmax (PSMATV50). In the present study, we analyzed the suitability of this approach comparing 18F-PSMA-1007 with 68Ga-PSMA-11 PET/CT scans and the potential of PSMATV50 for the prediction of overall survival (OS) in patients before 177Lu-PSMA radioligand therapy (RLT). Moreover, PSMATV50 was integrated into the PSMA PET/CT consensus statement as well as RECIP 1.0, and the prognostic value of these response classification systems was compared. Methods: This retrospective study included 70 patients with metastatic castration-resistant prostate cancer undergoing PSMA RLT. Thirty-three patients were monitored by 68Ga-PSMA-11 PET/CT, and 37 patients by 18F-PSMA-1007 PET/CT. PET/CT scans before (baseline) and at the end of PSMA RLT after 2–4 cycles (follow-up) were separately analyzed by 2 readers. PSMATV50 at baseline and its change at the time of follow-up (ΔPSMATV50, expressed as a ratio) were correlated with OS using Cox proportional-hazards regression. The results of both subgroups were compared. The integration of ΔPSMATV50 in existing response classification systems was evaluated. To assess and compare the discriminatory strength of these classification systems, Gönen and Heller concordance probability estimates were calculated. Results: PSMATV50 determination was technically feasible in all examinations. A higher PSMATV50 at baseline and a higher ΔPSMATV50 were strongly associated with a shorter OS for both 68Ga-PSMA-11 (PSMATV50: hazard ratio [HR] of 1.29 [95% CI, 1.05–1.55], P = 0.009; ΔPSMATV50: HR of 1.83 [95% CI, 1.08–3.09], P = 0.024) and 18F-PSMA-1007 (PSMATV50: HR of 1.84 [95% CI, 1.13–2.99], P = 0.014; ΔPSMATV50: HR of 1.23 [95% CI, 1.04–1.51], P = 0.03). Response assessment provided high discriminatory power for OS for the PSMA PET/CT consensus statement (concordance probability estimate, 0.73) as well as RECIP 1.0 (concordance probability estimate, 0.74). Conclusion: PSMATV50 and ΔPSMATV50 proved to be predictive of OS not only for 68Ga-PSMA-11 but also for 18F-PSMA-1007 PET/CT scans. Subsequent integration of ΔPSMATV50 into the PSMA PET/CT consensus statement and RECIP 1.0 provided equally high prognostic value for both classification systems.
Prostate-specific membrane antigen (PSMA)–targeting PET/CT has remarkably advanced the staging of patients with prostate cancer and has proven to be superior to conventional imaging (1). Additionally, PSMA PET/CT is also frequently used in the context of PSMA radioligand therapy (RLT) to assess sufficient PSMA expression of prostate cancer manifestations before treatment and to evaluate therapy response (2). However, systematic response evaluation of PSMA RLT is still based primarily on biochemical parameters, that is, serum prostate-specific antigen level (3) and nonstandardized qualitative PSMA PET/CT assessment. With the emerging clinical significance of PSMA RLT in the management of prostate cancer (4,5), particularly highlighted by the recently completed phase III study (6) and the recent approval of 177Lu-PSMA-617 by the American Food and Drug Administration (7), an implementation of a reproducible and systematic evaluation system for PSMA PET/CT is of high interest.
Fanti et al. recently published PSMA PET progression criteria for general response assessment of prostate cancer treatments, integrating PSMA PET/CT with clinical and biochemical parameters (8). Although not yet clinically implemented, these response assessment criteria were validated for PSMA RLT as reproducible and highly predictive of overall survival (OS) in a retrospective analysis by our study group (9) and have been updated by a recently published PSMA PET/CT consensus statement (10). Therein, the definition of partial response (PR), stable disease, and progressive disease (PD) of patients with polymetastatic disease is based on a change in whole-body tumor volume on PSMA PET/CT. Another promising evaluation system is the recently suggested Response Evaluation Criteria in PSMA PET/CT (RECIP 1.0) (11), which showed the highest reproducibility and prognostic accuracy in a comparison of 5 different response criteria (including PSMA PET progression criteria) (12). Several quantification methods for the assessment of whole-body tumor volume have been proposed, mainly using a liver-based threshold on 68Ga-PSMA-11 PET/CT (13–17). However, this approach cannot be directly transferred to 18F-PSMA-1007 PET/CT because of the hepatobiliary excretion of this tracer (18). In contrast, Seifert et al. (19) recently introduced a semiautomatic method using a 50% threshold of lesion SUVmax to assess the whole-body tumor volume (PSMATV50). As this threshold approach is independent of the different physiologic tracer uptake of PSMA-targeting radiopharmaceuticals, it could also be applicable to 18F-PSMA-1007 PET/CT.
The primary objective of this retrospective analysis was to assess the feasibility and the prognostic value of PSMATV50 for OS in both 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT in patients with advanced metastatic castration-resistant prostate cancer. In a second step, PSMATV50 was integrated into the PSMA PET/CT consensus statement and RECIP 1.0 criteria, and the secondary objective was to compare the prognostic value of these response classification systems.
MATERIALS AND METHODS
Patient Population
All patients treated with at least 1 cycle of PSMA RLT between July 2015 and October 2020 at our department were screened for eligibility. PSMA PET/CT scans were performed before PSMA RLT (baseline PSMA PET/CT) and at the end of therapy after either 2 or 4 cycles (follow-up PSMA PET/CT). For inclusion, both baseline and follow‐up PET/CT had to be performed in-house with the same PSMA radioligand (68Ga‐PSMA‐11 or 18F‐PSMA‐1007) but not necessarily on the same PET/CT scanner. Another inclusion criterion was the availability of survival data. Patients without a follow‐up PET/CT scan (i.e., in cases of clinical progression) were excluded from the analysis. The local institutional review board approved this study (approval 251/17), and all subjects gave written informed consent. PSMA RLT was performed on a compassionate-use basis according to individual tumor board recommendations (20,21).
Treatment and Imaging Protocol
PSMA RLT was performed according to current guidelines (20). The standard protocol consisted of infusion of 6.0 GBq of 177Lu-PSMA-617 (n = 59) or 177Lu-PSMA-I&T (n = 11) (which are of comparable efficacy (20,22)) at an interval of 6–8 wk, with treatment response assessed by PSMA PET/CT and laboratory data 6–8 wk after the second cycle. Depending on the response to therapy, PSMA RLT was either continued with 2 additional cycles, following the same protocol, or discontinued if there was a good response or clear progression (based on clinical decision). Whole‐body PSMA PET scans were acquired after 1 h (68Ga‐PSMA‐11) or 2 h (18F‐PSMA‐1007) from mid thigh to skull, typically using a scan duration of 2 min per bed position. Contrast‐enhanced diagnostic CT with dose modulation (120 kVp, 100–400 mAs) was performed. Scans were acquired on a Vereos digital PET/CT (Philips), a Gemini TF 64 PET/CT (Philips), or a Gemini TF 16 Big‐Bore PET/CT (Philips) device. Images were reconstructed with a vendor‐specific iterative reconstruction algorithm (blob ordered-subset time-of-flight) with 3 iterations and 9 subsets (relaxation parameter, 0.35) and a voxel size of 2 × 2 × 2 mm (Vereos digital) or with 3 iterations and 33 subsets (relaxation parameter, 0.35) and a voxel size of 2 × 2 × 2 mm (Gemini TF 64 and Gemini TF 16 Big‐Bore). The spatial resolution of the reconstructed PET images was about 5 mm (Vereos) and 7 mm (both Gemini TF devices) in full width at half maximum, respectively. Prostate-specific antigen levels were assessed directly before administration of PSMA RLT and at follow-up PSMA PET/CT.
Semiautomatically Quantified Tumor Volume Assessment
68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT scans at baseline and follow-up were retrospectively analyzed by 2 readers with 2 and 4 y of PSMA PET/CT reader experience. Fiji (23) and the Beth Israel plugin (24) were used to calculate whole-body tumor volume. Autosegmentation was used for automatic delineation of PET-positive lesions, that is, regions of interest. Regions of interest comprising tissue with physiologic radioligand uptake were carefully removed manually, whereas regions of interest for pathologic lesions not detected by autosegmentation were added manually by the reader. In accordance with Seifert et al. (19), individual lesions were volumetrically assessed by applying a lesion-specific threshold of 50% of the local SUVmax to each region of interest. The summed volumes of all lesions correspond to the whole-body PSMA tumor volume (PSMATV50, measured in mL). The change in PSMATV50 at follow-up PSMA PET/CT compared with the baseline assessment (ΔPSMATV50, expressed as a ratio) was calculated for all individuals.
Response Assessment Using the PSMA PET/CT Consensus Statement and RECIP 1.0
PET/CT images were retrospectively analyzed by the readers using the local PACS system DeepUnity Diagnost (Dedalus HealthCare). According to RECIP 1.0, the appearance of at least 1 new lesion was noted. After the assessment of interobserver agreement, a final consensus was reached and used for further comparisons in combination with ΔPSMATV50. Since all patients in our cohort were polymetastatic, progression according to the PSMA PET/CT consensus statement was based solely on an increase in ΔPSMATV50 of more than 30% and not on the appearance of new lesions as well, which is proposed for an early stage of disease. The definitions of disease progression for the respective criteria are summarized in Table 1.
Response Assessment According to PSMA PET/CT Consensus Statement (10) and RECIP 1.0
Statistical Analysis
SPSS, version 24.0.0.0 (IBM), was used for statistical analyses. Data are presented as mean ± SD and range. An unpaired t test was used to assess differences between the characteristics of the 2 subgroups (68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT). An OS landmark analysis was performed, monitoring the interval between the follow‐up PSMA PET/CT and either death or last follow‐up. OS is presented as median with the 95% CI. To assess interrater reliability for tumor volume assessment, intraclass correlation coefficient was used, using single measures, calculated with a 2-way mixed-effect model (intraclass correlation coefficient(3,1)) for absolute agreement. For qualitative response assessment, the Cohen κ was used to assess interrater reliability. The association of PSMATV50 and ΔPSMATV50 with OS for each radiotracer, as well as for the sum of all patients, was analyzed by Cox proportional-hazards regression using hazard ratios (HRs). To assess and compare the discriminatory strength of response classification systems, Gönen and Heller concordance probability estimates excluding ties (25) were calculated using R, version 4.2.1, whereas the χ2 test with Cramér V was used to assess their cross-table correlation. Corresponding Kaplan–Meier-curves were analyzed by log-rank tests. P values of less than 0.05 were considered statistically significant.
RESULTS
Between July 2015 and October 2020, 70 of 120 patients receiving PSMA RLT were included in this retrospective analysis. Mean age was 73.0 ± 8.3 y (range, 53–90 y). In total, 196 treatment cycles were administered, with 43 patients receiving only 2 cycles and 27 patients receiving 4 cycles. PSMA RLT was stopped after 2 cycles because of either a clear response (clinical, biochemical, or PET/CT; n = 27) or respective progression (n = 16). The mean and cumulative administered activity was 5.8 ± 0.8 GBq (range, 3.0–7.5 GBq) per cycle and 16.1 ± 6.0 GBq (6.1–24.6 GBq), respectively. Detailed patient characteristics are given in Table 2. Previous 223Ra-dichloride therapy was significantly more prevalent among patients examined with the formerly used 68Ga-PSMA-11 (P = 0.007), as this treatment has in large part been replaced by PSMA RLT. Apart from that, no significant differences in age, time since initial diagnosis, serum prostate-specific antigen level before PSMA RLT, Gleason score, or other previous treatments were found for the 2 subgroups (68Ga-PSMA-11 and 18F-PSMA-1007; P > 0.05). The interval between baseline PSMA PET/CT and application of the first cycle was 45 ± 26 d (range, 2–126 d). The time from baseline PSMA PET/CT to the end of therapy and to follow-up PSMA PET/CT was 195 ± 66 d (range, 84–346 d) and 48 ± 9 d (range, 29–69 d), respectively. In 33 patients, PSMA RLT was monitored using 68Ga-PSMA-11 PET/CT, and 37 patients were examined using 18F-PSMA-1007 PET/CT. Mean prostate-specific antigen levels at follow-up PSMA PET/CT were 227.0 ng/mL (range, 0.1–1,111.0 ng/mL) for the 68Ga-PSMA-11 group and 394.5 ng/mL (range, 0.15–5,000.0 ng/mL) for the 18F-PSMA-1007 group.
Patient Characteristics at Baseline (n = 70)
Determination of PSMATV50 was technically feasible in all 140 examinations (Fig. 1). Interrater agreement for PSMATV50 at both baseline and follow-up PSMA PET/CT was high for both 68Ga-PSMA-11 PET/CT (intraclass correlation coefficient(3,1), 0.92 [95% CI, 0.87–0.95]; P < 0.001) and 18F-PSMA-1007 PET/CT (intraclass correlation coefficient(3,1), 0.82 [95% CI, 0.72–0.88]; P < 0.001). Detailed PSMATV50 and interrater data for both radiopharmaceuticals at all time points are given in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org). Interrater agreement on response assessment was very high for both PSMA PET/CT consensus statement (98.6%; Cohen κ = 0.97, P < 0.001) and RECIP 1.0 (95.7%; Cohen κ = 0.93, P < 0.001).
Maximum-intensity projections of 68Ga-PSMA-11 (A) and 18F-PSMA-1007 (B) PET scans of patients with metastasized prostate cancer before PSMA RLT. PSMA-positive prostate cancer lesions were delineated semiautomatically and highlighted in blue. Lesion-specific threshold of 50% was used. Intensity-scale bar is SUV.
Median follow-up (reverse Kaplan–Meier estimator) was 25.0 mo (95% CI, 12.7–37.3 mo) from follow-up PSMA PET/CT. Median OS was 9.0 mo (95% CI, 8.0–10.0 mo), with 24 patients (34%) being alive at the last follow-up. There were no therapy-related deaths documented.
Association of PSMATV50 and ΔPSMATV50 with OS
A higher PSMATV50 at baseline PSMA PET/CT was significantly associated with a shorter OS for patients examined with 68Ga-PSMA-11 PET/CT (n = 33; HR, 1.29 [95% CI, 1.05–1.55]; P = 0.009) and 18F-PSMA-1007 PET/CT (n = 37; HR, 1.84 [95% CI, 1.13–2.99]; P = 0.014). An increase in PSMATV50 at the follow-up PSMA PET/CT, resulting in a higher ratio of ΔPSMATV50 (>1.0), was strongly associated with a shorter OS for both 68Ga-PSMA-11 PET/CT (n = 33; HR, 1.83 [95% CI, 1.08–3.09]; P = 0.024) and 18F-PSMA-1007 PET/CT (n = 37; HR, 1.23 [95% CI, 1.04–1.51]; P = 0.03). Taking both radiopharmaceuticals together, the same association with OS was found for baseline PSMATV50 (n = 70; HR, 1.48 [95% CI, 1.16–1.90]; P = 0.002) and for ΔPSMATV50 (n = 70; HR, 1.23 [95% CI, 1.02–1.49]; P = 0.032).
Integration of ΔPSMATV50 into the PSMA PET/CT Consensus Statement and RECIP 1.0 Criteria
The PSMA PET/CT consensus statement classified 56% (n = 39) of patients as PD, 24% (n = 17) as stable disease, and 20% (n = 14) as PR. Kaplan–Meier analysis revealed a strong association between PD and shorter median OS compared with non-PD (PR and stable disease) in median OS (8.0 mo [95% CI, 6.7–9.3 mo] vs. 21.0 mo [95% CI, 17.9–40.2 mo], P = 0.002; Fig. 2A) and risk of death (HR, 2.65 [95% CI, 1.37–5.12]; P = 0.004). At least 2 new lesions appeared in 57% of patients (n = 40), but this parameter was not integrated into the response classification because all patients were in the polymetastatic stage of disease. New lesions were seen predominantly in patients classified as PD (n = 31, 78%), were less frequent in patients with stable disease (n = 7, 17%), and were seen in only 2 cases of PR (5%). According to RECIP 1.0, 53% (n = 37) of all patients were categorized as PD, whereas 31% (n = 22) and 16% (n = 11) were classified as stable disease and PR, respectively. Of the 48 patients with at least 1 new lesion on either PET or CT, 77% (n = 37) were classified as PD; the remaining 23% (n = 11) were classified as stable disease. Kaplan–Meier analysis again showed a large difference in median OS for patients with PD compared with non-PD (8.0 mo [95% CI, 6.2–9.8 mo] vs. 18.0 mo [95% CI, 6.1–19.9 mo], P = 0.001; Fig. 2B) and a significantly higher risk of death (HR, 2.69 [95% CI, 1.42–5.11]; P = 0.002). A Kaplan–Meyer analysis comparing all 3 response groups for both classification systems is given in Supplemental Figure 1.
Kaplan–Meier curves of OS of all patients (n = 70) classified by PSMA PET/CT consensus statement (A) and RECIP 1.0 (B).
Correlation between both systems was very high (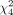 = 90.3, P < 0.001, Cramér V = 0.80). Correspondingly, concordance probability estimates were high for both the PSMA PET/CT consensus statement, at 0.73 (SE, 0.07), and RECIP 1.0, at 0.74 (SE, 0.06). A corresponding cross table comparison is shown in Table 3.
= 90.3, P < 0.001, Cramér V = 0.80). Correspondingly, concordance probability estimates were high for both the PSMA PET/CT consensus statement, at 0.73 (SE, 0.07), and RECIP 1.0, at 0.74 (SE, 0.06). A corresponding cross table comparison is shown in Table 3.
Comparison of Response According to PSMA PET/CT Consensus Statement (10) and RECIP 1.0
DISCUSSION
68Ga-PSMA-11 PET/CT evaluation at baseline before PSMA RLT showed a significant association between an increase in PSMATV50 and shorter OS, which is in line with the findings of Seifert et al. (19), despite the use of a different software solution. For 18F-PSMA-1007 PET/CT, PSMATV50 before PSMA RLT was also a prognostic biomarker for OS. PSMATV50 should be validated as a prognostic biomarker before other systemic treatment options (i.e., docetaxel or olaparib) and might serve as a decision support for treatment eligibility of patients. Furthermore, the change in PSMATV50 from baseline to follow-up PSMA PET/CT after the end of PSMA RLT was strongly associated with OS for both tracers. In summary, irrespective of the PSMA-targeting radiopharmaceutical used, PSMATV50 appears to be not only a suitable imaging-based biomarker for a response prediction before PSMA RLT but also a robust response assessment parameter after PSMA RLT, independent of the number of administered cycles. Thus, ΔPSMATV50 could be integrated into existing response classifications and used for systematic, quantitative, and reproducible response assessment, comparable to, for example, RECIST (26) for CT, which is necessary for the use of PSMA PET/CT in clinical trials. In addition, a lesion-specific percentage threshold (and its whole-body summary) may be suitable for different PSMA-targeting radiopharmaceuticals established in clinical practice (e.g., 68Ga-PSMA-11, 18F-PSMA-1007, 18F-DCFPyL PSMA, or 18F-rh-PSMA-7) since it does not depend on the (slightly) different (27) physiologic distribution of the tracers (28).
ΔPSMATV50 data of all patients were used to assess end-of-treatment response to PSMA RLT according to the PSMA PET/CT consensus statement and RECIP 1.0. The correlation between the classification systems was high. Both provided strong discriminatory power for OS between progressive and nonprogressive disease. The exclusion of laboratory criteria and focus on PET data alone, as well as the emphasis on change in tumor volume shared by both classification systems, allow for a simple and highly predictive risk assessment. Interestingly, the appearance of a new PSMA PET–positive lesion, which is part of RECIP 1.0, was obviously without impact in this advanced, polymetastatic disease stage. Further research is necessary to compare these classification systems in an early stage of disease. Notably, 1 patient with a relatively short survival of 4 mo, who showed an overall decrease in tumor volume of more than 30% and 2 new PET-negative liver metastases at follow-up, was subsequently categorized as PR according to the PSMA PET/CT consensus statement. Although the overall shift in focus away from the occurrence of new lesions as a solitary criterion prevents overestimation of PD and appears beneficial (12), a solely PET-based definition of new lesions seems disadvantageous compared with RECIP 1.0 and should be reviewed.
The present analysis has some limitations. First, it is inherently limited by its retrospective design. Second, the number of treatment cycles varied between 2 and 4. However, for each individual patient, the end of therapy was not defined by a fixed number of cycles but rather was determined by either disease progression or the maximum achievable therapy response. Third, the comparison of the 2 subgroups (68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT) is not based on a matched-pair analysis. However, no significant differences in characteristics between the 2 subgroups were found, and the groups were thus considered comparable. Fourth, the software solution used for segmentation (23,24) was developed for 18F‐FDG PET and not for PSMA PET. Nevertheless, assessment of PSMATV50 by 68Ga-PSMA-11 PET/CT before PSMA RLT was confirmed to be a prognostic imaging-based marker in line with the findings by Seifert et al. (19). In addition, PSMATV50 was found to be valid for 18F-PSMA-1007 PET/CT. Last, despite the semiautomatic approach of PSMATV50 assessment, the present study still relied on manual deletion of physiologic tracer uptake, resulting in multiple manual adjustments, such as in the delineation of liver metastases (found in only 3% of patients). Broadly available software solutions based on, for example, user-independent deep-learning artificial intelligence could overcome this time-consuming process (∼5–10 min per scan), achieve a high repeatability of tumor volume assessment (17), and facilitate clinical adaptability.
CONCLUSION
This study presents PSMATV50 as a prognostic biomarker for OS before PSMA RLT, as well as its potential as a quantitative end-of-treatment response marker for patients undergoing PSMA RLT. Applying a semiautomatic approach, we found that PSMATV50 and ΔPSMATV50 were predictive of OS not only for 68Ga-PSMA-11 but also for 18F-PSMA-1007 PET/CT scans. Subsequent integration of ΔPSMATV50 in the PSMA PET/CT consensus statement and RECIP 1.0 provided an equally high prognostic value for both classification systems. Further research is necessary to compare the strength of these classification systems in an early stage of disease.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is semiautomatic, percentage-threshold–based whole-body tumor volume assessment in PSMA PET/CT a feasible and meaningful parameter for systematic response assessment of PSMA RLT?
PERTINENT FINDINGS: For both 68Ga-PSMA-11 or 18F-PSMA-1007—individually as well as together—PSMATV50 at baseline and its change at the end of PSMA RLT were significant prognostic markers for OS. Integration of PSMATV50 in the PSMA PET/CT consensus statement and RECIP 1.0 provided high prognostic value for both classification systems.
IMPLICATIONS FOR PATIENT CARE: Response assessment using change in PSMATV50 can complement and possibly enhance existing PSMA PET/CT response assessment criteria.
ACKNOWLEDGMENT
We thank Dr. Lars Frings, Medical Center, University of Freiburg, for his statistical counsel and advice.
Footnotes
Published online Oct. 27, 2022.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication June 6, 2022.
- Revision received October 13, 2022.










