Visual Abstract
Abstract
In light of increasing health-care costs, higher medical expenses should be justified socioeconomically. Therefore, we calculated the effectiveness and cost effectiveness of PET using the radiolabeled amino acid O-(2-18F-fluoroethyl)-l-tyrosine (18F-FET) compared with conventional MRI for early identification of responders to adjuvant temozolomide chemotherapy. A recently published study in isocitrate dehydrogenase wild-type glioma patients suggested that 18F-FET PET parameter changes predicted a significantly longer survival already after 2 cycles whereas MRI changes were not significant. Methods: To determine the effectiveness and cost effectiveness of serial 18F-FET PET imaging, we analyzed published clinical data and calculated the associated costs from the perspective of the German Statutory Health Insurance system. Based on a decision-tree model, the effectiveness of 18F-FET PET and MRI was calculated—that is, the probability to correctly identify a responder as defined by an overall survival of at least 15 mo. To determine the cost effectiveness, the incremental cost effectiveness ratio (ICER) was calculated—that is, the cost for each additionally identified responder by 18F-FET PET who would have remained undetected by MRI. The robustness of the results was tested by deterministic and probabilistic Monte Carlo sensitivity analyses. Results: Compared with MRI, 18F-FET PET increased the rate of correctly identified responders to chemotherapy by 26%; thus, 4 patients needed to be examined by 18F-FET PET to identify 1 additional responder. Considering the respective costs for serial 18F-FET PET and MRI, the ICER resulted in €4,396.83 for each additional correctly identified responder by 18F-FET PET. Sensitivity analyses confirmed the robustness of the results. Conclusion: In contrast to conventional MRI, the model suggests that 18F-FET PET is cost-effective in terms of ICER values. Considering the high cost of temozolomide, the integration of 18F-FET PET has the potential to avoid premature chemotherapy discontinuation at reasonable cost.
Glioblastomas represent a pheno- and genotypically defined group of brain tumors characterized by a rapid and infiltrative growth resulting in a dismal prognosis for affected patients (1). The standard of care consists of resection, followed by radiotherapy with concomitant and adjuvant temozolomide chemotherapy, according to the EORTC-NCIC 22981/26981 trial (2). To evaluate treatment effects, contrast-enhanced anatomic MRI is the most widely used tool to assess response to chemoradiation and adjuvant chemotherapy. However, the diagnostic performance of standard contrast-enhanced MRI is insufficient to identify treatment-related changes such as pseudoprogression (3–8), with an accuracy of approximately 50% (3). For example, MRI signal changes (e.g., an increase in the extent of contrast enhancement, newly diagnosed contrast-enhancing lesions, or an increase in signal alterations on fluid-attenuated inversion recovery sequences) may be related to infection or neuroinflammation, ischemia, demyelination, or treatment-related effects related to radiotherapy or chemoradiation with alkylating agents. All these changes may be difficult to distinguish from actual tumor progression and may impact patient care.
Several studies have indicated that assessment of metabolic tumor activity by PET using the radiolabeled amino acid O-(2-18F-fluoroethyl)-l-tyrosine (18F-FET) is both helpful and superior to conventional MRI for the detection of treatment-related changes after chemoradiation with temozolomide in glioma patients (3,9,10). In addition, a recent study by Ceccon et al. (11) investigated the value of serial 18F-FET PET in glioma patients for early assessment of treatment response to adjuvant chemotherapy with temozolomide. In that study, 41 newly diagnosed glioma patients after resection or biopsy and chemoradiation with temozolomide underwent 18F-FET PET imaging before initiation (baseline) of adjuvant chemotherapy with temozolomide and after the second cycle (follow-up). The authors concluded that, in contrast to MRI, a metabolic decrease in static 18F-FET PET parameters from baseline to follow-up significantly predicted both a prolonged progression-free survival (PFS) and overall survival (OS), thus allowing identification of responders to adjuvant temozolomide early after treatment initiation.
Nevertheless, the integration of 18F-FET PET in the care of glioma patients is associated with additional costs that have to be weighed against relevant clinical benefits for affected patients. In recent years, only a few studies have addressed the topic of the cost effectiveness of 18F-FET PET in the care of glioma patients, which contrasts with the considerable evidence confirming its usefulness. In detail, 18F-FET PET has already been proven to be cost-effective for surgical target selection (12,13) and for assessing the response to radiotherapy with concomitant temozolomide (14) or bevacizumab (15) in glioma patients.
Considering the diagnostic improvements and additional costs of 18F-FET PET compared with conventional MRI, the already published data of Ceccon et al. (11) were evaluated regarding the effectiveness of serial 18F-FET PET scans to identify responders to adjuvant temozolomide chemotherapy and cost effectiveness. This analysis was performed from the perspective of the statutory health insurance system in Germany. To the best of our knowledge, this was the first study investigating the effectiveness and cost effectiveness of serial 18F-FET PET imaging in the care of glioma patients after adjuvant chemotherapy with temozolomide.
MATERIALS AND METHODS
Input Data
The clinical value of 18F-FET PET compared with conventional MRI for identifying responders to adjuvant temozolomide chemotherapy was published by Ceccon et al. (11). In that study, 41 adult patients (mean age, 52 ± 13 y) with newly diagnosed and histomolecularly characterized glioma (glioblastoma, 90%) were included. The institutional review board approved the current study, and all subjects gave written informed consent to their participation in the study and evaluation of their data for scientific purposes. After resection or stereotactic biopsy, all patients completed radiotherapy (60 Gy) with concomitant and adjuvant temozolomide chemotherapy over 6 cycles according to the EORTC/NCIC 22981/26981 trial (2). After chemoradiation, all patients underwent both 18F-FET PET and MRI within 7 d before adjuvant temozolomide initiation and after the second cycle of adjuvant temozolomide. MRI changes at the first follow-up compared with baseline were assigned according to the Response Assessment in Neuro-Oncology (RANO) criteria (16). For the evaluation of imaging data, static 18F-FET PET parameters such as tumor-to-brain ratios and metabolic tumor volumes were calculated (17). We concluded that MRI changes (according to RANO criteria) did not have any predictive value for PFS and OS. In contrast, a change in static 18F-FET PET biomarkers such as maximum tumor-to-brain ratio and metabolic tumor volume from baseline to follow-up predicted a significantly longer PFS and OS, thus enabling early identification of responders and nonresponders to adjuvant temozolomide chemotherapy.
Decision-Tree Model for Comparison of Effectiveness
A decision-tree model was developed to compare the effectiveness of 18F-FET PET and MRI, that is, the probability of correctly identifying a responder. As described previously (12–14,18), this model was constructed: patients were divided into responders and nonresponders depending on individual neuroimaging findings on 18F-FET PET and MRI (Fig. 1). Chance node 1 indicated the probability of a patient’s being a responder or a nonresponder according to maximum tumor-to-brain ratio changes. Chance node 2 indicated this probability concerning MRI changes according to RANO criteria. The subsequent chance nodes 3–6 assigned each of the 4 groups of PET and MRI responders and nonresponders to the patients’ outcomes. In the study by Ceccon et al. (11), the response was associated with a PFS of at least 9 mo and an OS of at least 15 mo. We defined the probability of correct identification of a responder to adjuvant chemotherapy with temozolomide as the primary outcome of our model.
Decision-tree model for assessing effectiveness of 18F-FET PET and MRI to identify responders to adjuvant chemotherapy with temozolomide based on PFS ≥ 9 mo and OS ≥ 15 mo. Thirty-eight patients underwent serial 18F-FET PET, and 40 patients underwent serial MRI. Chance nodes 1 and 2 divide into responders or nonresponders according to PET and MRI criteria (i.e., maximum tumor-to-brain ratio ≤ or > 0%, and stable disease or progressive disease according to RANO criteria, respectively). Chance nodes 3–6 further divide into true and false responders and true and false nonresponders. # = corresponding likelihood (1 − P); N1, N2, N3, N4, N5, and N6 = chance nodes 1, 2, 3, 4, 5, and 6, respectively; TMZ = temozolomide.
Cost Calculation
The costs were calculated from the perspective of the German statutory health insurance system. As this insurance usually does not cover 18F-FET PET costs in the care of glioma patients, the costs for both 18F-FET PET and conventional MRI were calculated on the basis of the medical fee schedule for care outside the statutory health insurance scheme (http://www.e-bis.de/goae/defaultFrame.htm) to provide an equal and consistent comparison of the cost.
The costs taken into consideration for 18F-FET PET were as follows (procedure’s index number in parenthesis; €1.00 = ∼$1.02 at time of manuscript preparation): patient consultation, €10.72 (1); report on diagnostic findings, €17.43 (75); intravenous injection, €9.38 (253); scintigraphy of the brain, €125.91 (5430); PET with quantitative analysis, €786.89 (5489); and tracer production costs, €616.
For MRI, the costs were as follows: patient consultation, €10.72 (1); physical examination, €10.72 (5); report on diagnostic findings, €17.43 (75); high-pressure intravenous injection, €40.23 (346); surcharge for perfusion imaging, €75.19 (3051); MRI with 3-dimensional and apparent diffusion coefficient reconstruction requiring substantial technical effort, €641.16 (5700); additional MRI series with 3-dimensional and apparent diffusion coefficient reconstruction requiring substantial technical effort, €145.72 (5731); and surcharge for computer analysis, €46.63 (5733).
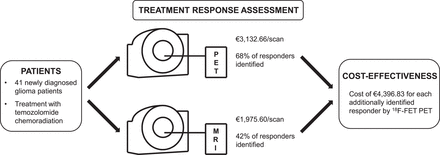
Thus, the neuroimaging cost was estimated at €1,566.33 for 1 18F-FET PET scan and €987.80 for 1 MRI scan. As the assessment of response comprised 2 scans, the total costs for each patient resulted in €3,132.66 for 18F-FET PET and €1,975.60 for MRI.
The overall cost of concomitant radiochemotherapy followed by 6 cycles of temozolomide was approximately €30,000 (19,20).
Cost Effectiveness
The difference in cost between 2 serial 18F-FET PET and MRI scans divided by the incremental effectiveness (IE) to correctly detect a responder to adjuvant chemotherapy with temozolomide resulted in the incremental cost effectiveness ratio (ICER):
Sensitivity Analyses
Deterministic and probabilistic sensitivity analyses were performed to test the robustness of the calculated effectiveness.
In particular, the deterministic sensitivity analysis evaluated the impact of each independent variable (chance nodes 1–6) on the resulting ICER. For this, we used CIs already applied in previous studies, which evaluated the cost effectiveness of 18F-FET PET in glioma patients undergoing chemoradiation with concomitant temozolomide (14) or antiangiogenic therapy using bevacizumab (Table 1) (15).
Chance Node Intervals and Corresponding IE and ICER in 1-Way Deterministic Sensitivity Analysis for Decision-Tree Regarding OS and PFS
For probabilistic sensitivity analysis, a Monte Carlo simulation was performed using 10,000 sets of random values for the independent variables (chance nodes 1–6). The distribution of these random values was defined by the mean of our decision tree and the SD, which was set according to the respective CI of the deterministic sensitivity analysis, similar to Baguet et al. (Table 2) (14).
Input Variables Used in Monte Carlo Analysis
For each set of random values, we determined the IE and ICER. Moreover, 18F-FET PET and MRI costs were modeled by a γ-distribution with the mean of the difference in cost between serial 18F-FET PET and MRI scans and an SD of 50%. Results from the probabilistic sensitivity analysis for effectiveness values were displayed by mean, median, SD, 95% CI, minimum, and maximum values and the 2.5th, 10th, 90th, and 97.5th percentiles. All calculations, figures, and simulations were performed using R software (https://www.r-project.org/).
RESULTS
Effectiveness
The decision-tree model for OS and PFS revealed that serial 18F-FET PET increased the number of correctly identified responders to adjuvant temozolomide chemotherapy compared with MRI alone. With regard to OS, the proportion of responders additionally identified by 18F-FET PET was 26% higher than the proportion identified by MRI (18F-FET PET responders, 68%; MRI responders based on RANO criteria, 42%). For PFS, the IE of 25% was similar (18F-FET PET responders, 68%; MRI responders based on RANO criteria, 43%). Thus, to identify 1 responder by 18F-FET PET, 4 patients had to be examined (number needed to examine, 3.8 for OS; 3.9 for PFS).
Cost Calculation
The ICER resulted in €4,396.83 for OS and €4,568.90 for PFS for each responder identified by 18F-FET PET but not by MRI.
Sensitivity Analyses
The resulting ICER for the chance node intervals of the deterministic sensitivity analysis are presented in Table 1. Figure 2 shows the corresponding Tornado diagrams. The range of ICER values was €2,805.52–€10,224.32 for OS and €2,864.73–€11,205.90 for PFS. Chance nodes 1 and 2 showed by far the most significant impact regarding the minimum and maximum ICER values, as a direct result of their wider variability.
Tornado diagram of ICER of 18F-FET PET for identification of responders to adjuvant chemotherapy with temozolomide based on OS and PFS. ICERs were calculated by applying upper and lower interval values, as shown in Table 1, onto chance nodes 1–6.
The results of the probabilistic sensitivity analysis showed both a narrow distribution around the mean and a close relation to the calculated IE and ICER values of the decision tree for OS (mean IE, 26% [CI, 24%–27%]; mean ICER, €4,437.41 [CI €4,337.24–€4,919.98]) and PFS (mean IE, 25% [CI 23%–26%]; mean ICER, €4,610.24 [CI €4,470.05–€5,119.95]) (Table 3; Fig. 3). This close relation confirmed the robustness and reliability of the calculated values of the decision tree.
Statistics Resulting from Monte Carlo Analysis (10,000 Samples) for Effectiveness of 18F-FET PET and MRI for Identification of Responder to Adjuvant Chemotherapy with Temozolomide
Distribution of results from Monte Carlo analysis (dots) about IE of 18F-FET PET for identification of 1 responder to adjuvant chemotherapy with temozolomide compared with MRI based on OS ≥ 15 mo and PFS ≥ 9 mo. x-axis depicts increase in likelihood of correct identification of responder as outcome (IE). y-axis depicts γ-modulated difference in cost between serial 18F-FET PET and MRI scans. Values with IE < 0 and > 0.6 are not shown (1% of values).
DISCUSSION
The main finding of the present study is that 18F-FET PET is effective and cost-effective for early identification of responders to adjuvant chemotherapy with temozolomide compared with standard MRI in patients with malignant glioma. Our results are based on the responsiveness to chemotherapy as a surrogate since this responsiveness considerably influences further treatment planning in these patients. This particularly applies to clinically equivocal situations in which treatment-related changes such as pseudoprogression on MRI after chemoradiation with temozolomide might lead to a discontinuation of a benefitting chemotherapy. Thus, a premature and more aggressive treatment regimen based on the false assumption of nonresponsiveness to temozolomide, with the risk of severe side effects, reduced survival, and a decrease in health-related quality of life, can potentially be avoided.
Considering the overall cost of concomitant radiochemotherapy followed by 6 cycles of temozolomide (∼€30,000) (19,20), the expense for 18F-FET PET for treatment assessment seems to be cost-effective. This particularly applies when considering the total costs for patient care and a potential cost reduction if an unnecessary, more aggressive treatment can be avoided. Thus, a neuroimaging approach combining both conventional MRI and 18F-FET PET has the potential to improve the respective strengths of each imaging modality at acceptable cost.
To the best of our knowledge, this was the first study evaluating the cost effectiveness of serial 18F-FET PET for assessing response to adjuvant chemotherapy with temozolomide. Recently, a study from Baguet et al. investigated the cost effectiveness of 18F-FET PET for assessment of treatment response in glioma patients after radiotherapy with concomitant temozolomide (14). Similar to our results, the authors found almost equal IE values for PFS and OS and concluded that 18F-FET PET might be cost-effective for that purpose. Nevertheless, there are several differences from the present study. In particular, the authors investigated the cost effectiveness of 18F-FET PET for identifying nonresponders to radiotherapy with concomitant temozolomide chemotherapy. Thus, an earlier phase of treatment was analyzed, and the respective decision trees were based on the clinical assumption of nonresponsiveness. Furthermore, the authors investigated 18F-FET PET from the perspective of the Belgian health-care system, resulting in different costs for 18F-FET PET. Another difference is the size of the patient samples, which was larger in our study (41 vs. 25 patients).
Other studies evaluated the cost effectiveness of additional 18F-FET PET compared with MRI alone for biopsy site selection for glioma diagnosis (12) and bevacizumab response assessment in patients with progressive malignant glioma (15). In analogy to our study, both studies concluded that 18F-FET PET is cost-effective concerning the analyzed clinical scenarios. Compared with our results, the respective ICERs were higher, that is, €9,114 for 1 additional correct glioma diagnosis after 18F-FET PET–guided biopsy (12) and €8,145 to identify 1 additional responder to bevacizumab (15). This difference is due to a decreased IE for 18F-FET PET as compared with MRI with regard to Heinzel et al. (12). Moreover, whereas the ICER in the mentioned studies (12,15) reflected the cost of adding 18F-FET PET in the diagnostic workup, the ICER in the present study reflects the result of comparing both imaging strategies (18F-FET and MRI) with their respective cost effectiveness ratios. This limits the meaningfulness of comparing the ICERs of the mentioned studies with the present results. In patients with brain metastases, a further study investigated the cost effectiveness of 18F-FET PET for the differentiation of brain metastasis relapse from radiotherapy-induced changes (18). The ICER of that study was similar to our results. Taken together, our results confirm previous studies suggesting that 18F-FET PET is cost-effective in the care of patients with brain malignancies.
One limitation of the present study is that the decision trees for PFS and OS were based on merely 1 study relying on longitudinal within-group comparisons. Though these groups comprised a large number of prospectively followed patients, additional studies with prospective designs are warranted, particularly given the paucity of research regarding this crucial medical–economic topic.
CONCLUSION
This study suggested that 18F-FET PET is cost-effective for early treatment response assessment in glioma patients after chemotherapy with temozolomide and helps to improve patient care at acceptable costs.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 18F-FET PET cost-effective for early identification of responders to adjuvant chemotherapy with temozolomide in glioma patients?
PERTINENT FINDINGS: On the basis of published data, 18F-FET PET increased the rate of correctly identified responders by 26% as compared with MRI, resulting in a cost of €4,396.83 for each additionally identified responder. This appears to be cost-effective, particularly considering the high cost of temozolomide chemotherapy.
IMPLICATIONS FOR PATIENT CARE: The integration of 18F-FET PET may improve patient care at reasonable cost.
Footnotes
Published online Apr. 14, 2022.
- © 2022 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication January 2, 2022.
- Revision received March 9, 2022.