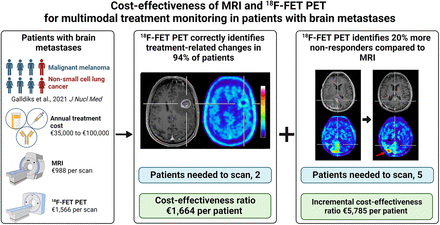
Visual Abstract
Abstract
PET using the radiolabeled amino acid O-(2-[18F]fluoroethyl)-l-tyrosine (18F-FET) has been shown to be of value for treatment monitoring in patients with brain metastases after multimodal therapy, especially in clinical situations with equivocal MRI findings. As medical procedures must be justified socioeconomically, we determined the effectiveness and cost-effectiveness of 18F-FET PET for treatment monitoring of multimodal therapy, including checkpoint inhibitors, targeted therapies, radiotherapy, and combinations thereof in patients with brain metastases secondary to melanoma or non–small cell lung cancer. Methods: We analyzed already-published clinical data and calculated the associated costs from the German statutory health insurance system perspective. Two clinical scenarios were considered: decision tree model 1 determined the effectiveness of 18F-FET PET alone for identifying treatment-related changes, that is, the probability of correctly identifying patients with treatment-related changes confirmed by neuropathology or clinicoradiographically using the Response Assessment in Neuro-Oncology criteria for immunotherapy. The resulting cost-effectiveness ratio showed the cost for each correctly identified patient with treatment-related changes in whom MRI findings remained inconclusive. Decision tree model 2 calculated the effectiveness of both 18F-FET PET and MRI, that is, the probability of correctly identifying nonresponders to treatment. The incremental cost-effectiveness ratio was calculated to determine cost-effectiveness, that is, the cost for each additionally identified nonresponder by 18F-FET PET who would have remained undetected by MRI. One-way deterministic and probabilistic sensitivity analyses tested the robustness of the results. Results: 18F-FET PET identified 94% of patients with treatment-related changes, resulting in €1,664.23 (€1.00 = $1.08 at time of writing) for each correctly identified patient. Nonresponders were correctly identified in 60% by MRI and in 80% by 18F-FET PET, resulting in €3,292.67 and €3,915.83 for each correctly identified nonresponder by MRI and 18F-FET PET, respectively. The cost to correctly identify 1 additional nonresponder by 18F-FET PET, who would have remained unidentified by MRI, was €5,785.30. Conclusion: Given the considerable annual cost of multimodal therapy, the integration of 18F-FET PET can potentially improve patient care while reducing costs.
In patients with late-stage cancer, brain metastases develop in up to 40% of cases, worsening the patient’s clinical status and prognosis, frequently necessitating a treatment change. In these patients, oligometastasis resection, stereotactic radiosurgery, whole-brain radiotherapy, and conventional cytotoxic chemotherapy are common treatment options (1). In recent years, the advent of checkpoint inhibitor immunotherapy and targeted therapies has amplified treatment options by enhancing local tumor control, thereby improving the patient’s prognosis. Monitoring the effects of these therapies on brain metastases by contrast-enhanced anatomic MRI has proved challenging regarding the differentiation of treatment-related changes from brain metastasis relapse (2). Notably, MRI signal changes may reflect treatment-related changes, especially in combination with radiotherapy, or metastasis relapse.
Considering the limited specificity of anatomic MRI for monitoring treatment effects in patients with brain metastases, PET has increasingly been used to metabolically assess cerebral lesions. Although PET using 18F-FDG is the tracer of choice for numerous diagnostic approaches in patients with cancer, the Response Assessment in Neuro-Oncology Working Group has recommended the use of PET with radiolabeled amino acids such as O-(2-[18F]fluoroethyl)-l-tyrosine (18F-FET), as it shows an excellent lesion-to-background ratio due to the relatively low uptake in the normal brain parenchyma (3).
Moreover, a recent study by our group (4) has suggested that assessing the metabolic activity in patients with brain metastases using 18F-FET PET is both helpful and superior to conventional MRI for monitoring the treatment effects. In particular, we investigated the value of 18F-FET PET in patients with brain metastases secondary to malignant melanoma or non–small cell lung cancer for treatment monitoring of multimodal therapy, including immune checkpoint inhibitors, targeted therapies, radiotherapy, or combinations thereof. We concluded that static 18F-FET PET parameters are valuable for differentiating treatment-related changes from brain metastasis relapse and identifying responders with a longer stable clinical course after treatment. Notably, anatomic MRI could not provide this essential clinical information alone.
Nevertheless, integrating 18F-FET PET in the care of patients with brain metastases is associated with additional costs that must be weighed against relevant clinical benefits for affected patients. In recent years, only 1 study has addressed the cost-effectiveness of 18F-FET PET for the differentiation of treatment-related changes from brain metastasis relapse after radiotherapy (5). In contrast to that study, the patients included in our analysis were additionally treated with concurrently or subsequently applied checkpoint inhibitors, targeted therapies, or combinations thereof. These agents, particularly when used in combination with radiotherapy, may render MRI findings highly variable, resulting in difficulties in terms of interpretation (6). Other studies focused on the cost-effectiveness of 18F-FET PET in glioma patients for surgical target selection or response assessment after different treatment options (7–10). Recently, another study analyzed the cost-effectiveness of the somatostatin receptor PET ligand 68Ga-DOTATATE for postresection radiotherapy planning in meningioma patients (11). Overall, these studies consistently suggested that PET is cost-effective regarding the analyzed clinical scenario.
Considering the diagnostic improvements and additional costs of 18F-FET PET compared with anatomic MRI, our group’s already-published study (4) was evaluated regarding the effectiveness and cost-effectiveness of 18F-FET PET in identifying treatment-related changes and nonresponders after multimodal therapy, including immune checkpoint inhibitors, targeted therapies, radiotherapy, or combinations thereof. This analysis was performed from the perspective of the statutory health insurance system in Germany. To our knowledge, this is the first study investigating the effectiveness and cost-effectiveness of 18F-FET PET imaging for managing this patient group.
MATERIALS AND METHODS
Input Data
Our group’s study on the value of 18F-FET PET for treatment monitoring of multimodal therapy in patients with brain metastases secondary to malignant melanoma or non–small cell lung cancer was published in 2021 (4). In that retrospective study, 40 adults (mean age, 59 ± 13 y) with 107 contrast-enhancing lesions on cerebral MRI were included. Of those 40 patients, 2 patients had to be excluded from further analysis (one patient was lost to follow-up, and the other patient died and the death was not cancer-related), resulting in 38 patients. The institutional review board approved this study, and all subjects gave written informed consent for study participation and evaluation of their data for scientific purposes. The patients were predominantly heavily pretreated with different combinations of immune checkpoint inhibitors, targeted therapies (e.g., BRAF inhibitors), and radiotherapy, and all underwent both MRI and 18F-FET PET during subsequent follow-up for treatment monitoring. A single 18F-FET PET scan was additionally performed on a subgroup of patients (n = 27) to differentiate treatment-related changes from brain metastasis relapse when anatomic MRI resulted in equivocal findings. In this group, the static 18F-FET PET parameter mean tumor-to-brain ratio (threshold, 1.95) was found to differentiate best between treatment-related changes and brain metastasis relapse (accuracy, 85%). Clinical verification of the 18F-FET PET imaging diagnosis was based on a stable clinical course during subsequent follow-up and either a neuropathologic diagnosis or a clinicoradiologic diagnosis using the Response Assessment in Neuro-Oncology criteria for immunotherapy (12). In the remaining patients (n = 11), 18F-FET PET and MRI were performed both at baseline and at follow-up to assess response to treatment. Metabolic response was defined as a relative reduction in the mean tumor-to-brain ratio of 10% or more for 18F-FET PET, a threshold that we found separates best responders from nonresponders using receiver-operating-characteristic curve analyses (4). A stable clinical course for at least 6 mo served as the reference for validating the respective imaging diagnosis. We concluded that 18F-FET PET imaging added valuable information for the differentiation of treatment-related changes from brain metastasis relapse when prior MRI remained inconclusive and treatment response evaluation was beyond the information provided by MRI alone.
Decision Tree Models for Assessment of Effectiveness
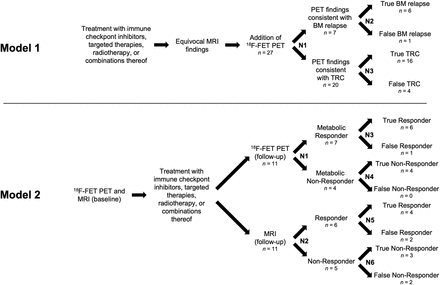
Similar to earlier studies (7–10,13), 2 decision tree models were developed for both analyzed subgroups to assess effectiveness (Fig. 1). Each model integrates the temporal sequence of the applied therapies and neuroimaging and assigns the patients according to their respective imaging findings and, subsequently, their definite diagnosis.
Model 1 (upper panel): Decision tree for assessing effectiveness of additional 18F-FET PET for differentiating treatment-related changes (TRC) from brain metastasis (BM) relapse after multimodal therapy. Twenty-seven patients underwent 18F-FET PET. N1 divides patients into those diagnosed with brain metastasis relapse or treatment-related changes according to 18F-FET PET criteria (i.e., mean tumor-to-brain ratio of more or less than 1.95, respectively). N2 and N3 assign both groups to patients’ outcomes based on both clinical course during subsequent follow-up and either neuropathologic diagnosis or Response Assessment in Neuro-Oncology criteria for immunotherapy. Model 2 (lower panel): Decision tree model for assessing effectiveness of 18F-FET PET and MRI to identify nonresponder to multimodal therapy based on stable clinical course for <6 mo. Eleven patients underwent serial 18F-FET PET and MRI. N1 and N2 represent chance nodes to be responder or nonresponder according to 18F-FET PET and MRI criteria (i.e., relative reduction or increase in mean tumor-to-background ratio of 10% for 18F-FET PET, respectively; Response Assessment in Neuro-Oncology criteria for immunotherapy for MRI). N3–N6 divide each of 4 groups of 18F-FET PET and MRI responders (and nonresponders) into true and false responders (and nonresponders), respectively.
Model 1
After multimodal therapy and subsequent equivocal MRI findings, 18F-FET PET was additionally performed to differentiate between treatment-related changes and a relapse of brain metastases. Chance node 1 (N1) divided patients into groups depending on individual 18F-FET PET findings, and subsequent chance nodes N2 and N3 assigned both groups to the patient’s outcomes. We defined the probability of correct identification of treatment-related changes as the primary outcome of model 1.
Model 2
After baseline 18F-FET PET and MRI, and subsequent multimodal therapy, 18F-FET PET and MRI were performed at follow-up to assess response to treatment. Chance nodes divided patients into responders or nonresponders according to 18F-FET PET metabolic findings (N1) or MRI changes according to the Response Assessment in Neuro-Oncology criteria for immunotherapy (N2). The subsequent chance nodes N3–N6 assigned each of the 4 groups of 18F-FET PET and MRI responders (and nonresponders) to the patients’ outcomes. In contrast to model 1, model 2 was designed to compare the effectiveness of 18F-FET PET and MRI. We defined the probability of correct identification of a nonresponder to multimodal therapy as the primary outcome of model 2.
Cost Calculation
The costs were calculated from the perspective of the German statutory health insurance system. As the German statutory health insurance companies usually do not cover 18F-FET PET costs in the care of patients with brain metastases, the costs for both 18F-FET PET and conventional MRI were based on the medical fee schedule for care outside the statutory health insurance scheme (http://www.e-bis.de/goae/defaultFrame.htm) to provide an equal and consistent determination of the cost.
As described previously (10), the costs taken into consideration for 18F-FET PET were as follows: patient consultation, €10.72 (€1.00 = $1.08 at time of writing) (procedure index no. 1); report on diagnostic findings, €17.43 (procedure index no. 75); intravenous injection, €9.38 (procedure index no. 253); scintigraphy of the brain, €125.91 (procedure index no. 5430); and 18F-FET PET with quantitative analysis, €786.89 (procedure index no. 5489). Tracer production costs were €616.00. For MRI, the costs were as follows: patient consultation, €10.72 (procedure index no. 1); physical examination, €10.72 (procedure index no. 5); report on diagnostic findings, €17.43 (procedure index no. 75); high-pressure intravenous injection, €40.23 (procedure index no. 346); surcharge for perfusion imaging, €75.19 (procedure index no. 3051); MRI with 3-dimensional and apparent-diffusion-coefficient reconstruction requiring substantial technical effort, €641.16 (procedure index no. 5700); additional MRI series with 3-dimensional and apparent-diffusion-coefficient reconstruction requiring substantial technical effort, €145.72 (procedure index no. 5731); and surcharge for computer analysis, €46.63 (procedure index no. 5733).
Thus, the neuroimaging cost was estimated at €1,566.33 for 1 18F-FET PET scan and €987.80 for 1 MRI scan. In decision tree model 1, because the differentiation of treatment-related changes from brain metastasis relapse comprised a single 18F-FET PET scan, the cost for each patient was €1,566.33. In decision tree model 2, the assessment of response comprised, on average, 2 18F-FET PET and 2 MRI scans, resulting in total costs for each patient of €3,132.66 for 18F-FET PET and €1,975.60 for MRI.
Cost-Effectiveness
In decision tree model 1, the effectiveness of correctly identifying treatment-related changes after multimodal therapy was calculated for 18F-FET PET alone. Thus, the cost for 1 18F-FET PET scan divided by its effectiveness resulted in the cost-effectiveness ratio (CER):
In decision tree model 2, the effectiveness of correctly detecting a nonresponder to multimodal therapy was compared between 18F-FET PET and MRI (i.e., incremental effectiveness [IE]). Thus, the difference in cost between 2 serial 18F-FET PET and 2 MRI scans divided by the IE resulted in the incremental cost-effectiveness ratio (ICER):
Sensitivity Analyses
Deterministic and probabilistic sensitivity analyses were performed to test the robustness of the calculated effectiveness. In particular, 1-way deterministic sensitivity analysis evaluated the impact of each independent variable (model 1, N1–N3; model 2, N1–N6) on the resulting effectiveness and, thus, the CER and ICER. Because of a lack of previous studies evaluating the cost-effectiveness of 18F-FET PET in patients with brain metastases after multimodal therapy, available CIs already used in a comparable study, which evaluated the cost-effectiveness of 18F-FET PET for the differentiation of brain metastasis relapse from radiation-induced changes after radiotherapy, were applied to each variable (Table 1) (5). In model 2, because the calculated value for N4 was 100% (as shown in the corresponding decision tree), changing that chance node value within the deterministic sensitivity analysis resulted in theoretic values of more than 100%. Thus, the resulting IE and ICER based on these values are likewise considered theoretic.
Chance Node Intervals and Corresponding Effectiveness and CER in 1-Way Deterministic Sensitivity Analysis for Decision Tree Models 1 and 2
For probabilistic sensitivity analysis, a Monte Carlo simulation was performed using 10,000 sets of positive random values for the independent variables (model 1, N1–N3; model 2, N1–N6). The distribution of these random values was defined by the mean of the decision trees and SD of 18F-FET PET already used in the mentioned study (Table 2) (5). For each set of random values, we determined the effectiveness of 18F-FET PET alone (model 1) or the effectiveness of both MRI and 18F-FET PET and their respective difference (i.e., IE) (model 2). The CER and ICER were based on the effectiveness values. All results for the 97.5th percentile and maximum value of effectiveness of 18F-FET PET exceeded 100%, and should be considered theoretic. Meanwhile, when these values served as the basis for calculation of the CI (i.e., the interval between the 2.5th and 97.5th percentiles of distributions derived from the Monte Carlo simulations) of both effectiveness and cost-effectiveness, they were set to a ceiling value of 100%. Moreover, imaging costs were modeled by a γ-distribution with the mean cost for 1 18F-FET PET scan (model 1) or the difference in cost between 2 serial 18F-FET PET and MRI scans (model 2) and an SD of 50% of the corresponding mean. The probabilistic sensitivity analysis results for effectiveness values are displayed by mean, median, SD, 95% CI, and minimum and maximum values and by the 2.5th, 10th, 90th, and 97.5th percentiles. All calculations, figures, and simulations were performed using the statistical computing language and environment R (https://www.r-project.org/; https://readxl.tidyverse.org/) (14). The graphical abstract was created with BioRender.com.
Input Variables Used in Monte Carlo Analysis
RESULTS
Effectiveness
Decision tree model 1 revealed that 18F-FET PET correctly identified treatment-related changes after multimodal therapy in 94% of patients when MRI findings were equivocal. Thus, 2 patients had to be examined to identify 1 patient with treatment-related changes. Decision tree model 2 revealed that serial 18F-FET PET increased the number of correctly identified nonresponders to multimodal therapy compared with MRI. The proportion of nonresponders additionally identified by 18F-FET PET was 20% higher than by MRI (18F-FET PET metabolic nonresponders, 80%; MRI nonresponders according to the Response Assessment in Neuro-Oncology criteria for immunotherapy, 60%). Thus, 5 patients had to be examined to identify 1 additional nonresponder by 18F-FET PET.
Cost Calculation
For decision tree model 1, the CER resulted in a cost of €1,664.23 for each patient with treatment-related changes identified by 18F-FET PET alone. For decision tree model 2, the cost to correctly identify 1 nonresponder was €3,292.67 and €3,915.83 for MRI and 18F-FET PET, respectively. The ICER, that is, the cost to correctly identify 1 additional nonresponder by 18F-FET PET who would have remained unidentified by MRI, was €5,785.30.
Sensitivity Analyses
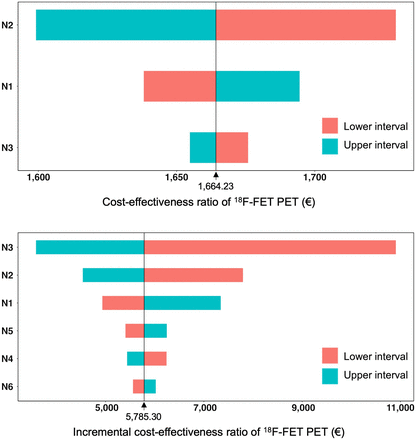
For decision tree model 1, the resulting CER for the chance node intervals of the deterministic sensitivity analysis is presented in Table 1. The upper panel in Figure 2 shows the corresponding tornado diagram. The range of CERs was €1,599.13–€1,729.33. The results of the probabilistic sensitivity analysis showed both a narrow distribution around the mean and a close relation to the calculated effectiveness and CERs of the decision tree (mean effectiveness, 94%; 95% CI, 86%–100%; mean CER, €1,664.61; 95% CI, €1,566.33–€1,814.31) (Table 3; upper panel in Fig. 3). This close relation confirmed the robustness and reliability of the calculated values of the decision tree.
Tornado diagrams of cost-effectiveness ratio of additional 18F-FET PET scans for identification of treatment-related changes (upper panel) and ICER of 18F-FET PET for identification of nonresponder (lower panel) after multimodal therapy. Cost-effectiveness ratios and ICERs were calculated by applying upper and lower interval values, as shown in Table 1, onto N1–N3 and N1–N6, respectively.
Statistics Resulting from Monte Carlo Analysis (10,000 Samples) for Effectiveness
Distribution of results from Monte Carlo analysis (dots) about effectiveness of additional 18F-FET PET for identification of treatment-related changes (upper panel) and IE of 18F-FET PET for identification of nonresponder (lower panel) after multimodal therapy. Note different scaling of axes. Margin values for effectiveness (upper panel, values < 0.85 and > 1.0, 5.0% of values; lower panel, values < 0 and > 0.5, 7.4% of values) are not shown.
For decision tree model 2, the resulting ICER for the chance node intervals of the deterministic sensitivity analysis is presented in Table 1. The lower panel in Figure 2 shows the corresponding tornado diagram. The range of ICERs was €3,585.31–€10,906.40. The results of the probabilistic sensitivity analysis showed both a narrow distribution around the mean and a close relation to the calculated IE and ICERs of the decision tree (mean IE, 21%; 95% CI, 17%–24%; mean ICER, €5,619.37; 95% CI, €4,894.90–€6,638.61) (Table 3; lower panel in Fig. 3). This close relation confirmed the robustness and reliability of the calculated values of the decision tree.
DISCUSSION
The main finding of the present study is that 18F-FET PET is both clinically effective and cost-effective in identifying treatment-related changes and nonresponders to multimodal therapy among patients with brain metastases secondary to malignant melanoma or non–small cell lung cancer.
Regarding decision tree model 1, our results are based on identifying treatment-related changes as a surrogate since this identification considerably influences further treatment planning in affected patients. This particularly applies to scenarios in which equivocal clinical and MRI findings after multimodal therapy might challenge the continuation of a benefitting treatment, possibly based on the false assumption of a brain metastasis relapse. In these cases, a premature change to a more aggressive treatment regimen, with the risk of severe side effects, reduced survival, and decreased health-related quality of life, can be avoided. Regarding decision tree model 2, our results are based on nonresponsiveness to treatment as a surrogate since this nonresponsiveness likewise heavily influences further treatment planning. This applies to clinical scenarios in which a noneffective and expensive treatment regimen can be discontinued, avoiding further treatment cost.
Given the considerable annual cost of immune checkpoint inhibitors or targeted therapies, ranging from approximately €35,000 to €100,000 in Germany (according to the Federal Joint Committee, https://www.g-ba.de), the expense for 18F-FET PET for treatment monitoring seems to be cost-effective. This particularly applies when considering the total costs for patient care and a potential cost reduction if ineffective treatment is discontinued for nonresponsiveness. Thus, a neuroimaging approach combining conventional MRI and 18F-FET PET can potentially improve the respective strengths of each imaging modality at acceptable cost.
To date, only a limited number of studies on the effectiveness and cost-effectiveness of 18F-FET PET are available, although there is considerable evidence confirming its usefulness in the care of patients with brain malignancies. To our knowledge, this is the first study evaluating the cost-effectiveness of 18F-FET PET for monitoring multimodal therapy in patients with brain metastases. A similar study (5) investigated the effectiveness and cost-effectiveness of 18F-FET PET for the differentiation of brain metastasis recurrence from radiation injury after radiotherapy in a similar clinical scenario to that shown in decision tree model 1 but without comedication using immune checkpoint inhibitors or targeted therapies. The authors concluded that 18F-FET PET appears to be cost-effective for that purpose. Compared with our results, the respective ICER was higher (€4.014, based on an “adjusted cost scenario” that was most similar to the present cost calculation) because of lower effectiveness of 18F-FET PET (42%). Nevertheless, in that study, the model assumed initial MRI findings suggestive of brain metastasis relapse for all patients, potentially prompting further invasive diagnostic procedures (e.g., stereotactic biopsy). In contrast, the present model assumed initially equivocal MRI findings. In the present study, this was reflected by the calculation of effectiveness for 18F-FET PET alone, hence limiting the meaningfulness of comparing the CERs of the mentioned study with the present results.
Other studies evaluated the cost-effectiveness of 18F-FET PET in the care of glioma patients for surgical target selection or response assessment after different treatment regimens (7–10). In brief, the respective calculated ICERs were roughly comparable to the present results, and the authors concluded that 18F-FET PET might likewise be cost-effective concerning the analyzed clinical scenario. However, the meaningfulness of a direct comparison of ICERs is limited, given the relevant differences in patients’ diagnoses and treatment conditions.
One limitation of the present study is that both decision tree models are based on merely 1 study relying on longitudinal within-group comparisons in a subgroup of predominantly heavily pretreated patients with brain metastases. For example, the relatively low number of included patients analyzed in model 2 may result in a greater degree of uncertainty in terms of ICER. Thus, the present results warrant confirmation by a comparable study with more patients. Another limitation is that the cost was calculated within the context of a specific, that is, German, health care system. As a consequence, the present results for the CERs and ICERs cannot directly be transferred to other countries because of national differences in health care systems and cost structures. On the other hand, the present results for the incremental effectiveness (i.e., the probability of correctly identifying patients with treatment-related changes) appear to be transferable to other countries because these depend predominantly on the information obtained from the respective neuroimaging modality. Thus, incremental effectiveness may help physicians choose the most appropriate neuroimaging approach during follow-up and considerably facilitate further cost evaluations from the perspective of non-German health economics. In addition, earlier studies evaluating the effectiveness and cost-effectiveness of 18F-FET PET for other applications in patients with brain tumors have used an equivalent approach (5,7–10), also from the perspective of the Belgian health care system (13).
CONCLUSION
This study suggests that 18F-FET PET is clinically effective and cost-effective for monitoring multimodal therapy in patients with brain metastases secondary to malignant melanoma or non–small cell lung cancer and improves patient care at acceptable costs.
DISCLOSURE
Norbert Galldiks received honoraria for lectures from Blue Earth Diagnostics and for advisory board participation from Telix Pharmaceuticals. Karl-Josef Langen and Felix M. Mottaghy received honoraria for consultancy service from Telix Pharmaceuticals. Philipp Lohmann received speaker honoraria from Blue Earth Diagnostics. No other potential conflict of interest relevant to this article was reported.
KEYPOINTS
QUESTION: Is 18F-FET PET cost-effective for multimodal treatment monitoring in patients with brain metastases secondary to malignant melanoma or non–small cell lung cancer?
PERTINENT FINDINGS: On the basis of published data, 18F-FET PET is cost-effective in identifying both treatment-related changes and nonresponders after multimodal therapy, including immune-checkpoint inhibitors, targeted therapy, radiotherapy, and combinations thereof.
IMPLICATIONS FOR PATIENT CARE: Regarding the considerable annual costs of treatments with immune checkpoint inhibitors or targeted therapies, the integration of 18F-FET PET may improve patient care and reduce costs.
Footnotes
Published online Apr. 25, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication September 23, 2023.
- Revision received March 13, 2024.