Abstract
68Ga-DOTA-JR11 is a somatostatin receptor subtype 2–specific antagonist used for PET/CT imaging. The purpose of this study was to compare 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT in patients with metastatic, well-differentiated neuroendocrine tumors. Methods: Patients with histologically proven, metastatic or unresectable, well-differentiated neuroendocrine tumors were prospectively recruited to this study. Each patient received an intravenous injection of 68Ga-DOTATATE (155 ± 52 MBq) on the first day and 68Ga-DOTA-JR11 (148 ± 52 MBq) on the second day. Whole-body PET/CT scans were performed at 40–60 min after injection on the same scanner. Physiologic normal-organ uptake, lesion numbers, and lesion uptake were compared. Results: Thirty-one patients were prospectively enrolled in the study. The SUVmax of the spleen, renal cortex, adrenal glands, pituitary glands, stomach wall, normal liver parenchyma, small intestine, pancreas, and bone marrow was significantly lower on 68Ga-DOTA-JR11 than on 68Ga-DOTATATE PET/CT (P < 0.001). 68Ga-DOTA-JR11 detected significantly more liver lesions (552 vs. 365, P = 0.001) but fewer bone lesions (158 vs. 388, P = 0.016) than 68Ga-DOTATATE. The target-to-background ratio of liver lesions was significantly higher on 68Ga-DOTA-JR11 (7.7 ± 5.4 vs. 3.4 ± 2.0, P < 0.001). 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT showed comparable results for primary tumors and lymph node metastases on both patient-based and lesion-based comparisons. Conclusion: 68Ga-DOTA-JR11 performs better in detecting liver metastases, with a better tumor-to-background ratio, whereas 68Ga-DOTATATE may outperform 68Ga-DOTA-JR11 in the detection of bone metastases. However, the lower somatostatin receptor subtype 2 affinity of 68Ga-DOTA-JR11 than of 177Lu-DOTA-JR11 may limit its role as a diagnostic pair for the theranostic approach with 177Lu-DOTA-JR11.
Somatostatin receptor (SSTR), especially SSTR subtype 2 (SSTR2), is the key target for the theranostic approach to neuroendocrine tumors (NETs). With different isotopes labeled, radiolabeled somatostatin analogs have been used clinically either for imaging or for peptide receptor radionuclide therapy (1–3). Since the approval of OctreoScan by the Food and Drug Administration in 1994, many 68Ga-labeled molecules for imaging purposes have emerged, such as DOTATATE, DOTATOC, and DOTANOC. SSTR PET/CT imaging plays an important role in the primary tumor detection, staging, and restaging of NETs. Furthermore, as the imaging half of theranostics, it provides key information in deciding whether patients are eligible for peptide receptor radionuclide therapy. All these mentioned agents, which are SSTR agonists, are internalized into tumor cells after the ligand–receptor interaction (4).
An important development in the field of SSTR targeting was the recent introduction of SSTR antagonists (5–10). The results for the first radiolabeled antagonists were published in 1996 by Bass et al. (11). Radiolabeled LM3, JR10, and JR11, the second generation of antagonists (12), have been developed and evaluated in patients with NETs (7). Despite lack of internalization, preclinical and clinical studies suggested that radiolabeled SSTR antagonists may perform better than agonists (5,6). They showed more favorable pharmacokinetics, better image contrast, higher tumor uptake, and better residence time. The possible reason is that antagonists can recognize more binding sites on receptors.
Recently, the first-in-humans study of 68Ga-DOTA-JR11, conducted by Krebs et al., showed good safety and biodistribution profiles in patients with metastatic NETs (13). Rapid tumor uptake, high tumor-to-background ratios, and rapid clearance from blood were demonstrated in the study. Nicolas et al. directly compared the sensitivity of 68Ga-NODAGA-JR11 and 68Ga-DOTATOC and found that antagonists were superior to agonists in sensitivity, lesion detection, and image contrast (14,15).
With antagonists, we now have an alternative to agonists. However, there is still not much evidence about the performance of PET/CT imaging with SSTR antagonists. Hence, we designed this prospective study to compare 68Ga-DOTATATE and 68Ga-DOTA-JR11 PET/CT in patients with metastatic, well-differentiated NETs.
MATERIALS AND METHODS
Study Design and Patient Population
This study was approved by the institutional review board of Peking Union Medical College Hospital, and all subjects gave written informed consent before study participation. Patients with histologically proven, metastatic or unresectable, well-differentiated NETs (G1 or G2) were prospectively and consecutively recruited to this study. To avoid the influence of radiolabeled somatostatin analog treatment on imaging, patients who had received long-acting radiolabeled somatostatin analog treatment within 4 wk before the study were excluded (16). The 2 PET/CT scans were conducted on 2 consecutive days.
68Ga-DOTATATE and 68Ga-DOTA-JR11 Preparation
Good-manufacturing-practice–grade precursors, DOTA-JR11 and DOTATATE, were supplied by CS Bio Co. and ABX GmbH, respectively. The radiolabeling was performed manually in a hot cell. Briefly, 68GaCl3 was eluted from a 68Ge/68Ga generator (Eckert and Ziegler) using 5 mL of 0.1 M hydrochloride acid. The eluate was added to a reaction vial containing the precursor (for DOTA-JR11, 80 μg; for DOTATATE, 40 μg) and dissolved in sodium acetate buffer, for a final pH of 4 for the reaction mixture. The mixture was heated to 100°C for 10 min. After being cooled to room temperature, the reaction mixture was diluted with 5 mL of water and then loaded onto a C18 light Sep-Pak cartridge (preconditioned with 10 mL of ethanol and 10 mL of water) and washed with normal saline to remove unincorporated radionuclide. Finally, the product was eluted off the cartridge with 75% ethanol solution, diluted with saline, and passed through a Millipore filer (0.22 μm, 25 mm) into a sterile product vial. The radiochemical purity of the final product was more than 95%.
68Ga-DOTATATE and 68Ga-DOTA-JR11 PET/CT Imaging
The study was performed on a time-of-flight PET/CT scanner (Polestar m660; SinoUnion Healthcare Inc.) on 2 consecutive days. Patients received an intravenous injection of 68Ga-DOTATATE (155 ± 52 MBq) on the first day and 68Ga-DOTA-JR11 (148 ± 52 MBq) on the second day. A low-dose CT scan (120 keV; 100 mAs; 1.3 pitch; 2.5-mm slice thickness; 0.5-s rotation time; estimated radiation dose, 9.0 mGy) from head to proximate thigh was obtained at 40–60 min after injection for anatomic localization and attenuation correction. PET scanning followed at 2 min/bed position with a 23-slice overlap. Images were reconstructed using ordered-subsets expectation maximization (2 iterations, 10 subsets, 192 × 192 matrix) and corrected for CT-based attenuation, dead time, random events, and scatter.
Adverse Event Monitoring
Vital signs (blood pressure, body temperature, and heart rate) and clinical symptoms were monitored and recorded within 2 h after injection according to version 4.03 of the Common Terminology Criteria for Adverse Events.
Image Interpretation and Data Analysis
The images were reviewed on MIM software (MIM Software Inc.). One experienced nuclear medicine expert (25 y of experience in nuclear medicine), masked to the patient and medical history, reviewed the images.
For normal tissues, the physiologic uptake of 68Ga-DOTATATE and 68Ga-DOTA-JR11 was compared in the following organs: spleen, renal cortex, adrenal glands, pituitary gland, stomach, normal liver parenchyma, small intestine, and pancreas (uncinate process). Regions of interest were drawn over the organs, excluding focal lesions. Meanwhile, any activity from adjacent organs such as renal pelvis and urinary bladder was avoided. The SUVmax (using body weight normalization) of the regions of interest in normal organs was recorded. In the case of bilateral organs such as adrenal glands and renal cortex, the average SUVmax was calculated.
Any focal accumulations of 68Ga-DOTATATE and 68Ga-DOTA-JR11 not explained by physiologic uptake were interpreted as focal lesions. Volumes of interest of focal lesions were segmented using PET Edge, a gradient-based segmentation algorithm (17). The number and SUVmax of focal lesions were recorded. For liver and splenic lesions, the relative uptake of focal lesions was quantified using target-to-background ratio, defined as SUVmax (lesion)/SUVmax (normal parenchyma). Comparative analysis of SUVmax and target-to-background ratio between 68Ga-DOTATATE and 68Ga-DOTA-JR11 was conducted for matched lesions only.
Statistical Analysis
Data were expressed as mean ± SD. The differences in SUVmax and target-to-background ratio between 68Ga-DOTA-JR11 and 68Ga-DOTATATE were evaluated using paired t tests (SPSS, version 22). Statistical comparison of the lesion numbers was conducted using sign tests. A P value of less than 0.05 was considered to indicate a statistically significant difference.
RESULTS
Thirty-one patients were prospectively enrolled in the study. Their clinical characteristics are summarized in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org). No patient received treatment between 68Ga-DOTATATE and 68Ga-DOTA-JR11 PET/CT. Both tracers were tolerated well by all patients. No adverse events were reported.
Biodistribution Comparison Between 68Ga-DOTATATE and 68Ga-DOTA-JR11 PET/CT
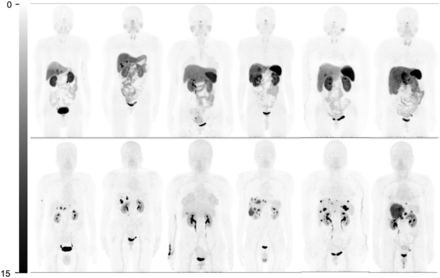
Unlike 68Ga-DOTATATE, 68Ga-DOTA-JR11 demonstrated minimal or mild uptake in almost all organs except the urinary tract (Fig. 1). The SUVmax of spleen, renal cortex, adrenal glands, pituitary glands, stomach wall, normal liver parenchyma, small intestine, pancreas, and bone marrow is shown in Table 1. The uptake by all listed normal organs was significantly lower on 68Ga-DOTA-JR11 PET/CT than on 68Ga-DOTATATE PET/CT (P < 0.001).
Comparison of whole-body maximum-intensity projections in 6 representative patients (patients 7, 8, 11, 14, 27, and 29 from left to right). Physiologic uptake is seen at pituitary gland, salivary glands, thyroids, adrenal glands, spleen (splenectomy in patients 7 and 8), and bowel on 68Ga-DOTATATE maximum-intensity projections (top). Nevertheless, these normal organs show none or very mild uptake on 68Ga-DOTA-JR11 maximum-intensity projections (bottom). Besides, 68Ga-DOTA-JR11 depicts more liver lesions than 68Ga-DOTATATE, with lower liver background.
Comparison of Normal-Organ Uptake Between 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT
Comparison of Tumor Detection Rates Between 68Ga-DOTATATE and 68Ga-DOTA-JR11 PET/CT
In total, 835 and 875 focal lesions were depicted on 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT, respectively (P = 0.152; Table 2).
Number of Lesions Found on 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT
On patient-based comparison, 68Ga-DOTA-JR11 demonstrated a higher detection ability for liver lesions (Fig. 1). Of 26 patients with liver metastases, 54% (14/26) showed more liver metastases on 68Ga-DOTA-JR11 than on 68Ga-DOTATATE, whereas 42% (11/26) demonstrated comparable results. Only 1 patient had fewer liver lesions detected on 68Ga-DOTA-JR11 PET/CT. For bone lesions, however, 68Ga-DOTA-JR11 was inferior to 68Ga-DOTATATE in 78% (7/9) of patients (Fig. 2).
Patient-based comparison of lesion detection.
On lesion-based comparison, 68Ga-DOTA-JR11 detected significantly more liver lesions (552 vs. 365, P = 0.001) but fewer bone lesions (158 vs. 388, P = 0.016; Fig. 3). 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT showed comparable results for primary tumors and lymph node metastases based on either patient-based or lesion-based comparison.
PET/CT images of 68Ga-DOTATATE and 68Ga-DOTA-JR11 in patient 23, with pancreatic NET and multiple liver, lymph node, and bone metastases. (A and B) 68Ga-DOTATATE maximum-intensity projection (A) shows many more bone lesions than 68Ga-DOTA-JR11 (B), whereas primary tumor, lymph node metastases, and liver metastases are comparable. (C and E) Transaxial fusion (C) and PET images (E) of 68Ga-DOTATATE show multiple bone lesions in pelvic bone. (D and F) Only one of them is positive with 68Ga-DOTA-JR11, on fusion (D) and PET (F) images.
Uptake Comparison Between 68Ga-DOTATATE and 68Ga-DOTA-JR11 PET/CT
For matched lesions, 68Ga-DOTA-JR11 demonstrated significantly lower uptake by all lesions (Table 3). The target-to-background ratio for liver lesions, however, was significantly higher on 68Ga-DOTA-JR11 than on 68Ga-DOTATATE (7.7 ± 5.4 vs. 3.4 ± 2.0, P < 0.001). The 2 matched splenic lesions also showed a higher target-to-background ratio on 68Ga-DOTA-JR11 PET/CT.
Uptake of Matched Lesions on 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT
DISCUSSION
Our study prospectively compared the lesion detection rates between an SSTR antagonist, 68Ga-DOTA-JR11, and an agonist, 68Ga-DOTATATE, in a single group of patients. 68Ga-DOTA-JR11 had a higher rate than 68Ga-DOTATATE in the detection of liver metastases. For bone lesions, however, 68Ga-DOTA-JR11 was inferior to 68Ga-DOTATATE.
68Ga-DOTA-JR11 showed an overall lower tumor uptake than 68Ga-DOTATATE, for 2 possible reasons. The first reason lies in the different SSTR2 affinities of 68Ga-DOTA-JR11 and 68Ga-DOTATATE. 68Ga-DOTATATE has a much higher SSTR2 affinity than 68Ga-DOTA-JR11 (50% inhibitory concentration [IC50] is 0.2 vs. 29 nmol/L) (7). This difference is likely to have a negative impact on tumor uptake of 68Ga-DOTA-JR11, which is in fact worse than that of 68Ga-DOTATATE. An additional reason for the lower tumor uptake of 68Ga-DOTA-JR11 might be SSTR2 saturation or internalization after injection of 40 μg of 68Ga-DOTATATE 24 h ahead of 68Ga-DOTA-JR11 PET/CT. Reubi et al. showed in human NET tissue less receptor binding of the SSTR2-specific antibody on the cell membrane after injection of 200 μg of octreotide (4). This lower binding might be relevant even after injection of only 40 μg of 68Ga-DOTATATE, because 68Ga-DOTATATE has a 10 times higher affinity for SSTR2 than octreotide (12). These 2 reasons explain not only the lower tumor uptake of 68Ga-DOTA-JR11 but also, at least in part, the inferiority of 68Ga-DOTA-JR11 in the detection of bone metastases.
Nicolas et al. prospectively compared 68Ga-NODAGA-JR11 and 68Ga-DOTATOC in the same patients and found comparable tumor uptake between the 2 tracers (P > 0.05 in all lesions) (14). The seemingly contradictory results may again be explained by the different SSTR affinities. 68Ga-NODAGA-JR11 has a SSTR2 affinity comparable to that of 68Ga-DOTATOC (IC50 is 1.2 vs. 2.5 nmol/L) and much higher than that of 68Ga-DOTA-JR11 (IC50 is 29 nmol/L), indicating that 68Ga-DOTA-JR11 might not be the ideal diagnostic pair for a theranostic approach with 177Lu-DOTA-JR11, as 177Lu-DOTA-JR11 has a much better SSTR2 affinity than 68Ga-DOTA-JR11 (IC50 is 0.73 vs. 29 nmol/L). Furthermore, Reidy-Lagunes et al. found a good objective response and progression-free survival after 1–2 treatment cycles with 177Lu-DOTA-JR11 in 20 patients with NETs, including 7 patients with bone metastases (18). It is likely that 68Ga-NODAGA-JR11 is the better diagnostic pair because it has an SSTR2 affinity similar to that of 177Lu-DOTA-JR11 (IC50 is 1.2 vs. 0.73 nmol/L). However, there have been no intrapatient comparative data between 68Ga-DOTA-JR11 and 68Ga-NODAGA-JR11, and further studies are warranted.
Compared with 68Ga-DOTATATE, 68Ga-DOTA-JR11 shows a superior lesion detection ability for liver metastases based on both patient-based and lesion-based comparison. In a prospective study, Nicolas et al. compared the sensitivity of 68Ga-NODAGA-JR11 and 68Ga-DOTA-TOC in metastatic NETs (14). They reported an overall higher sensitivity for 68Ga-NODAGA-JR11, which was mainly due to more liver lesions detected. Our study further supports the superiority of 68Ga-DOTA-JR11 over 68Ga-DOTATATE in liver lesion detection. This superiority is probably caused by lower liver-background uptake and more binding sites on SSTR receptors recognized by the antagonist. Nevertheless, our study showed that the bone lesion detection ability of 68Ga-DOTA-JR11 is remarkably inferior to that of 68Ga-DOTATATE. An imaging comparison of bone metastases using antagonists and agonists has not, to our knowledge, been previously reported. The low affinity of 68Ga-DOTA-JR11 to bone metastases might have been overlooked in previous studies. The preliminary results of peptide receptor radionuclide therapy using antagonists reported by Wild et al. found a 1.1–7.2 times higher tumor-to-kidney/bone marrow uptake ratio for 177Lu-DOTA-JR11 than for 177Lu-DOTATATE (19). Nevertheless, only 4 patients were included in that study, and no bone lesions were present. Bone metastases were also not specified in the study by the Nicolas group (14).
The results for 68Ga-DOTA-JR11 tumor uptake were comparable to those of a previous study (13) for bone (7.8 ± 5.4 vs. 6 ± 3) and lymph node metastases (14.4 ± 10.3 vs. 14 ± 20) but lower for liver lesions (18.6 ± 12.5 vs. 25 ± 22). A possible reason is that we decided to include lesions regardless of the size criteria as long as they were identifiable on PET images. This decision significantly increased the number of liver lesions detected (552 in 26 patients vs. 30 in 20 patients), which decreased the average SUVmax since small lesions tend to have relatively lower uptake due to the partial-volume effect (20). In addition, saturation and internalization of SSTR2 receptors after 68Ga-DOTATATE injection may be another possible reason for low liver-lesion accumulation. The image contrast for liver lesions, however, was significantly higher on 68Ga-DOTA-JR11 PET/CT. This finding is, again, mainly due to the much lower uptake by normal liver parenchyma on 68Ga-DOTA-JR11 PET/CT (2.8 ± 0.9 vs. 9.7 ± 3.0, P < 0.001). The same is also true for splenic lesions, although no statistical comparison was conducted because of limited lesion numbers.
As a potential diagnostic companion for 177Lu-DOTA-JR11, the biodistribution of 68Ga-DOTA-JR11 in normal organs and tumor uptake is important in deciding whether patients are eligible for peptide receptor radionuclide therapy with 177Lu-DOTA-JR11. Our study demonstrated a more favorable biodistribution for 68Ga-DOTA-JR11 than for 68Ga-DOTATATE in patients with metastatic NETs, with minimal or mild uptake in almost all organs except the urinary tract. The low background activity provided an excellent image contrast, especially in liver, which is the predominant site of metastases in patients with gastroenteropancreatic NETs (21). Lower uptake was also observed in renal cortex and bone marrow. However, that finding does not imply that renal and bone marrow toxicity is lower with 177Lu-DOTA-JR11 than with 177Lu-DOTATATE; the SSTR2 affinity profile varies between 68Ga-DOTA-JR11 and 177Lu-DOTA-JR11. Furthermore, measurement of radiotracer uptake 40–60 min after injection supplies limited information to make any dose estimation. In fact, Wild et al. showed in a prospective crossover comparison of 177Lu-DOTA-JR11 and 177Lu-DOTATATE in the same patient no higher kidney or bone marrow dose with 177Lu-DOTATATE than with 177Lu-DOTA-JR11 (19). At the same time, tumor dose was higher with 177Lu-DOTA-JR11 than with 177Lu-DOTATATE. For organs with known SSTR expression, such as pituitary glands, adrenal glands, and spleen, there is either no or minimal uptake on 68Ga-DOTA-JR11 PET/CT. Besides, lack of uptake is also observed in stomach wall, small intestine, and the uncinate process of the pancreas, which usually demonstrate moderate uptake on 68Ga-DOTATATE PET/CT. This phenomenon was described in the previous study by Krebs et al. (13) and is currently not well understood. Irrespective of the cause, the low uptake in these organs is considered a major advantage of the antagonist over the agonist for potential detection of more lesions. It also helps to differentiate between physiologic uptake and real lesions.
In a previous study comparing 68Ga-NODAGA-JR11 and 68Ga-DOTATOC, the median time between the 2 scans was 34 d (interquartile range, 27.5–135 d) (14). Although NETs are relatively slow-growing tumors, disease progression during such a long time can still have a potential influence on imaging studies. Therefore, in our study, the 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT were done on 2 consecutive days to minimize the impact of disease progression. However, imaging on 2 consecutive days may also be a limitation of this study, because the 40 μg load of 68Ga-DOTATATE 24 h beforehand might be a cause of lower tumor uptake of 68Ga-DOTA-JR11 due to SSTR2 saturation and internalization. Besides, our study was limited by a lack of reference imaging studies, such as contrast-enhanced CT or MRI. Hence, the sensitivity of 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT cannot be calculated and further comparative studies are required.
CONCLUSION
68Ga-DOTA-JR11 is better able to detect liver metastases, with a better tumor-to-background ratio, whereas 68Ga-DOTATATE may outperform 68Ga-DOTA-JR11 in the detection of bone metastases. However, the lower SSTR2 affinity of 68Ga-DOTA-JR11 than of 177Lu-DOTA-JR11 may limit its role as a diagnostic pair for the theranostic approach with 177Lu-DOTA-JR11.
DISCLOSURE
This work was sponsored in part by the National Natural Science Foundation of China (81571713, 81601529), the CAMS Innovation Fund for Medical Sciences (2016-I2M-4-003), the CAMS Initiative for Innovative Medicine (2017-I2M-4-002, 2018-I2M-3-001), the Tianjin Natural Science Foundation (18JCQNJC11600), and the Tianjin Medical University Basic Research Foundation (2018KJ060). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does PET/CT with the SSTR antagonist 68Ga-DOTA-JR11 have a better ability to detect lesions than the agonist 68Ga-DOTATATE in patients with metastatic, well-differentiated NETs?
PERTINENT FINDINGS: Thirty-one patients with metastatic, well-differentiated NETs were prospectively recruited to compare the lesion detection ability of 68Ga-DOTA-JR11 PET/CT with that of 68Ga-DOTATATE PET/CT. 68Ga-DOTA-JR11 performs better in detecting liver metastases, whereas 68Ga-DOTATATE outperforms 68Ga-DOTA-JR11 in the detection of bone metastases.
IMPLICATIONS FOR PATIENT CARE: 68Ga-DOTA-JR11 is an optional alternative to SSTR agonists in patients with NETs, especially in liver-dominant metastases.
Acknowledgments
We thank all the patients who participated in this study. We also thank Yue Zhang (SinoUnion Healthcare Inc., China) for image acquisition and data collection and Dr. Chengyan Dong (GE Healthcare, China) for critical proofreading and figure suggestions.
Footnotes
Published online Nov. 1, 2019.
- © 2020 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication August 20, 2019.
- Accepted for publication October 10, 2019.