Abstract
Perturbation of thyroid iodide uptake is a well-documented side effect of the use of iodinated contrast media (ICM) administered intravenously. This side effect is thought to be mediated by free iodide in ICM formulations, but this hypothesis has never been formally proven. The aim of the present study was to assess the validity of this hypothesis. Methods: We used mass spectrometry analysis to quantify free-iodide contamination in ICM. Established cell lines expressing the Na/I symporter (NIS) were used to quantify the effect of ICM on iodide uptake. SPECT/CT was used to measure the in vivo uptake of 99mTc-pertechnetate and 123I in 2 NIS-expressing mouse tissues, thyroid and salivary glands. Scintiscans of ICM-naïve and ICM-administered patients were compared. Immunohistologic and Western blot analyses were performed to evaluate NIS protein expression in these organs. Results: Although free iodide was present in ICM formulations, in vitro uptake of iodide by NIS-expressing cells was not significantly affected by ICM. In mice, intravenous or sublingual administration of ICM led to a reduction in radiotracer uptake by the thyroid, accompanied by a dramatic reduction in NIS protein expression in this tissue. In the salivary glands, neither radiotracer uptake nor NIS protein expression was affected by ICM. The thyroid-selective effect of ICM was also observed in humans. Administration of potassium iodide as a source of free iodide led to a diminution of 99mTc-pertechnetate uptake in both mouse thyroid and mouse salivary glands. Altogether, these data rule out a direct intervention of free iodide in the perturbation of thyroid uptake and suggest a direct and selective effect of ICM on the thyroid. Conclusion: We demonstrated that ICM reduce thyroid uptake of iodide independently of free iodide. This effect is due to a specific and dramatic decrease in NIS expression in thyrocytes. These data cast serious doubt on the relevance of measuring urinary iodide concentration to evaluate the delay between ICM administration and radioiodine therapy in patients with differentiated thyroid carcinoma. Finally, the ability of ICM to perturb iodide uptake in the thyroid may be used in radioprotection.
Iodinated contrast media (ICM) are routinely administered to patients for x-ray imaging. In view of their widespread use, they can be considered safe. However, a well-documented side effect of intravenous ICM administration observed in most patients is compromise of diagnostic thyroid scintigraphy and radioiodine treatment of thyroid malignancies (1–5). For the latter, guidelines recommend delaying radioactive iodide treatment in patients who have been exposed to ICM (4,6–8). Mechanistically, this reduced iodide uptake by thyroid tissues in response to ICM is thought to be the result of injection of high amounts of free iodide contaminating the ICM formulation (9). Many studies have reported that urinary iodide concentration remains high for days or even weeks after ICM administration (10,11). This high urinary iodide concentration may reflect a high blood iodide content that could be consistent with the long-lasting effect of ICM on thyroid iodide uptake. The source of this free iodide may be free iodide associated with ICM (9) or free iodide released from ICM on injection (4,12). However, although this hypothesis is generally accepted, it has never, to our knowledge, been formally proven.
The protein responsible for iodide uptake is Na/I symporter (NIS), which is located at the basolateral membrane of some epithelial cells, including thyrocytes in the thyroid (13,14) and duct cells in the salivary gland (15). Ingestion of a large amount of potassium iodide (KI) leads to a high blood iodide content, which in turn dramatically reduces uptake of radioiodide and its surrogate, 99mTc-pertechnetate (99mTcO4−), in the thyroid and the salivary glands (16). However, this effect is transient, and a return to normal uptake capacities for both tissues is observed within 24 h after a single administration of a large amount of KI.
In this context, the aim of the present study was to determine experimentally whether the free iodide that contaminates ICM (9) or that can be released in vivo through deiodination of ICM in tissues (4,12) is responsible for the reduced iodide uptake by thyroid tissues. If either or both of these hypotheses are correct, Iomeron (Bracco Imaging) (as a prototype of ICM) would be expected to act in the same way as free iodide on iodide uptake in NIS-expressing cells and tissues.
MATERIALS AND METHODS
Animals
Eight-week-old female C57BL/6JRj mice were obtained from Janvier. The animals were treated in accordance with the French Agriculture Ministry guidelines, and the experiments were approved by the University of Nice Sophia Antipolis Animal Care User and Ethics Committee (Ciepal NCE/2014–211).
Contrast Agents
Experiments were performed using iomeprol (Iomeron 350; lot LP4557) and iodixanol (Visipaque 320 [GE Healthcare]; lot 125 78 776).
Cell Lines
The human colorectal cancer cell line HT-29 (HTB-38; American Tissue Culture Collection) was stably transfected with the expression plasmid pcDNA3.1-mNIS (murine NIS) (17), and a single clone with high functional expression of NIS was selected (18). The rat follicular thyroid cell line PCCL3 was obtained from Dr. Antonio De La Vieja (Madrid, Spain) and cultured as previously described (19). In vitro iodide uptake was measured as previously described (20).
Mass Spectrometry
Iomeron, Visipaque, and standard solutions of NaI were characterized by high-resolution mass spectroscopy in negative-mode electrospray. For the direct-infusion experiments, a flow of 5 μL/min was provided by a syringe pump (11 Plus; Harvard Apparatus) using a 500 μL/min syringe (Hamilton). The mass spectrometer (Q Exactive Plus; Thermo Fisher Scientific) was operated with an electrospray source for the direct-infusion method. The direct-infusion Orbitrap measurements were performed using the Ion Max source from Thermo Fisher Scientific and applying the following parameters: sheath gas flow, 15 arbitrary units; auxiliary gas flow, 5 arbitrary units; and capillary temperature, 275°C. The automatic gain control target was set to 106, and the maximum injection time was 50 ms. In the high-resolution measurements with a setting of 140,000 at m/z 200, 1 microscan was recorded. The spray voltage in negative mode was selected to be −2.5 kV.
In Vivo Administration of ICM to Mice
Intravenous and intraperitoneal administration was performed by injecting 100 μL of Iomeron diluted 50/50 (v/v) with phosphate-buffered saline (corresponding to 18 mg of iomeprol). Enteral administration was performed by gavage of 100 μL of Iomeron diluted 50/50 (v/v) with phosphate-buffered saline (corresponding to 18 mg of iomeprol). Sublingual administration was performed on anesthetized mice by 5 sublingual depositions of 5 μL of Iomeron, with a delay of 10 min in between. This procedure led to the administration of 9 mg of iomeprol.
Small-Animal SPECT/CT
99mTcO4− was obtained from a freshly eluted 99Mo/99mTc generator. 123I-NaI was purchased from IBA. The animals received 20 MBq of 99mTcO4− or 10 MBq of 123I− intraperitoneally. Thyroid and salivary gland uptake was measured at different times using a dedicated small-animal SPECT/CT scanner (eXplore speCZT CT120; GE Healthcare) as previously described (21). Reconstructed images were analyzed and quantified using AMIDE software (22). Tridimensional regions of interest were drawn manually around the thyroid and salivary glands as previously detailed (23,24). Uptake was expressed as percentage injected activity after decay correction (21).
Kinetics of 99mTcO4− or 123I-NaI Uptake Inhibition by ICM and KI
For ICM inhibition of 99mTcO4− or 123I-NaI uptake, SPECT/CT was performed on mice that had received 20 MBq of 99mTcO4− or 10 MBq of 123I-NaI (basal uptake, day 0). At the end of the scan, ICM was administered. At different times after ICM administration, the animals were injected with 20 MBq of 99mTcO4− or 10 MBq of 123I-NaI and new scans were obtained.
For KI inhibition of 99mTcO4− uptake, SPECT/CT was performed on mice that had received 20 MBq of 99mTcO4− (basal uptake). Twenty-four hours later, the animals were injected intraperitoneally with KI (9 mg in 180 μL). The animals were then injected 30 min later with 20 MBq of 99mTcO4− and scanned. Twenty-four hours later, the animals were injected with 20 MBq of 99mTcO4− and new scans were obtained.
Thyroid and Salivary Gland Uptake of 99mTcO4− in Humans
An ICM-naïve patient and a patient who had received an intravenous injection of Iomeron 350 2 wk before scintigraphy were chosen for study. On the day of the scintigraphy, the patients were injected intravenously with a 1 MBq/kg activity and 600-s scans were obtained 15 min later. The institutional review board approved this retrospective study, and the anonymized scans were obtained as part of routine medical examinations with consent of the patients.
Immunohistochemistry and Western Blot Analyses
After animals had been killed, the thyroids and salivary glands were dissected. Some thyroids and salivary glands were paraffin-embedded and cut into 4-μm-thick sections. The paraffin was then removed and the sections were rehydrated and subjected to an antigen retrieval treatment with a solution of citrate buffer, pH 6, using an automated station (PT Link; Dako). NIS immunostaining was performed as previously described (21). For Western blot analysis, thyroid and salivary gland membrane proteins were subjected to electrophoresis as previously described (21). Western blot analysis was performed with antibody 25 antimouse NIS, an affinity-purified rabbit immunoreactive serum fraction, or an anti-β-actin antibody (Sigma).
Statistical Analysis
Statistical analysis was performed using Prism (GraphPad Software). Dual comparisons were made using a Student t test, and comparisons between multiple conditions were analyzed using ANOVA. Statistical significance was set at a P value of less than 0.05.
RESULTS
Free Iodide Content of ICM
We first evaluated whether free iodide was present in commercially available ICM. Mass spectrometry analysis revealed that the concentrations of free iodide in Iomeron and Visipaque were in the range of 30 and 100 μM, respectively. The details of this dataset are available in Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org).
Effect of ICM on Uptake of Iodide in Cell Lines
We next examined whether iomeprol and its contaminating free iodide could affect iodide uptake in NIS-expressing cell lines. PCCL3 cells (a rat follicular thyroid cell line) were incubated with 125I in the presence of either saline buffer, Iomeron, NaI, or perchlorate (NaClO4). After 1 h, the cells were washed and the cellular 125I content was determined. As expected, both NaI and perchlorate inhibited iodide uptake in PCCL3 cells, but Iomeron failed to affect this uptake significantly (Fig. 1). Similar data were obtained using the HT29-NIS cell line (a human colorectal carcinoma cell line expressing NIS) (Supplemental Fig. 2).
Effect of Iomeron on iodide uptake of PCCL3 cells. PCCL3 cells were incubated for 1 h with 125I in equal volume of either saline, Iomeron (100 μL, corresponding to 71.4 mg of iomeprol in 3 mL of medium), NaI, or perchlorate. Cells were then rapidly washed with saline buffer and lysed. Aliquots of lysates were counted in γ-counter. Results are expressed as percentage uptake under control condition. Data are mean ± SEM of triplicates and are representative of 3 independent experiments. n.s = not statistically significant.
Effect of ICM on 99mTcO4− Uptake in Mice and Humans
We evaluated whether Iomeron could affect uptake of the iodide analog, 99mTcO4−, by the mouse thyroid and salivary glands. Basal uptake was measured before (T0) and after intravenous administration of Iomeron. Figure 2A shows that a dramatic reduction in radiotracer uptake by the thyroid was observed 24 h after Iomeron administration. This uptake capacity remained low for 8 d, and a recovery was observed on day 12. In the same animals, Iomeron administration failed to affect iodide uptake by another NIS-expressing tissue, the salivary glands (Fig. 2B). This differential effect was also obtained when 123I was used as the radiotracer (Supplemental Fig. 3). The differential uptake of 99mTcO4− by the thyroid and salivary glands was also observed using Visipaque (Supplemental Fig. 4). This observation suggests that ICM affect the mouse thyroid and the salivary glands differently. To evaluate whether this differential action is observed in humans, we compared 99mTcO4− uptake in the thyroid and salivary glands of an ICM-naïve patient (Fig. 3A) and a patient who had received an intravenous injection of Iomeron 2 wk before (Fig. 3B). Figure 3A shows the scintiscan of an ICM-naïve patient in whom both the thyroid and the salivary glands are taking up 99mTcO4−. The scintiscan of a patient treated with Iomeron shows radiotracer uptake in the salivary glands and a lack of fixation in the thyroid region (Fig. 3B).
Effect of Iomeron on uptake of 99mTcO4− by mouse thyroid and salivary glands. SPECT/CT of mice given 20 MBq of 99mTcO4− was performed. At end of scan, Iomeron was administered intravenously. One, 4, 8, 12, and 18 d later, animals were injected with 20 MBq of 99mTcO4− and new scans were obtained. Data are percentage radiotracer taken up by thyroid (A) and salivary glands (B) (n = 3 per condition). ***P < 0.001. %IA = percentage injected activity; n.s = not statistically significant.
Effect of Iomeron on uptake of 99mTcO4− by human thyroid and salivary glands. Scintiscans are of ICM-naïve patient (A) and patient given Iomeron 2 wk before scintigraphy (B). ANT = anterior; SG = salivary glands; T = thyroid.
Effect of KI on 99mTcO4− Uptake in Mice
A similar experiment was performed to evaluate the effect of KI on radiotracer uptake by the thyroid and salivary glands. Figure 4A shows that the ability of the thyroid to take up 99mTcO4− was reduced 30 min after intraperitoneal injection of KI. This capacity was recovered 24 h after injection. A similar pattern was observed when pertechnetate uptake to the salivary glands was measured (Fig. 4B). This dataset demonstrates that KI affects both thyroidal and salivary gland pertechnetate uptake.
Effect of KI on uptake of 99mTcO4− by mouse thyroid and salivary glands. SPECT/CT of mice given 20 MBq of 99mTcO4− was performed. Animals were injected intraperitoneally with KI (9 mg in 180 μL) and scanned 30 min later. Twenty-four hours later, animals were injected with 20 MBq of 99mTcO4− and new scans were obtained. Data are percentage radiotracer taken up by thyroid (A) and salivary glands (B) (n = 3 per condition). *P < 0.05. ***P < 0.001. %IA = percentage injected activity.
Effect of ICM on NIS Expression in Vivo
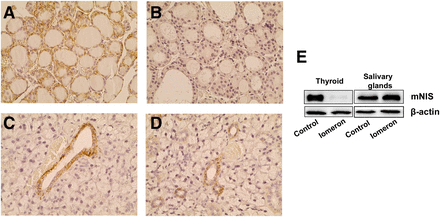
We next evaluated the effect of Iomeron on NIS expression in the thyroid and salivary glands. Immunohistologic analysis revealed that NIS expression was hardly detectable in the thyroid 4 d after Iomeron injection (Fig. 5B), as compared with control mice (Fig. 5A). By contrast, NIS expression was detectable in the ductal cells of the salivary glands of both control and Iomeron-treated mice (Figs. 5C and 5D). Semiquantitative Western blot analysis confirmed a dramatic decrease in NIS expression in the thyroid glands of Iomeron-treated mice compared with controls (Fig. 5E). No significant difference in NIS expression in the salivary gland was detected between the 2 groups.
Analysis of NIS expression in thyroid and salivary glands in response to Iomeron. Saline buffer (A and C) or Iomeron (B and D) was administered intravenously. Four days later, animals were culled and NIS expression in thyroid (A and B) and salivary glands (C and D) was analyzed by immunohistochemistry (n = 3 per group). Thyroid and salivary glands were also processed for Western blot analysis of NIS and β-actin expression (n = 2 per group).
Modes of Administration of ICM in Mice
We next compared the efficacy of different modes of administration of Iomeron on 99mTcO4− uptake by the thyroid and the salivary glands. Figure 6A shows that intravenous and sublingual administration of the ICM resulted in a marked reduction in radiotracer uptake (Fig. 6A). A similar effect was obtained with Visipaque (Supplemental Fig. 4A), whereas neither enteral nor intraperitoneal administration affected radiotracer uptake by the thyroid. Uptake to the salivary glands was not affected under any condition (Fig. 6B and Supplemental Fig. 4).
Evaluation of effect of different modes of administration of Iomeron on uptake of 99mTcO4− by thyroid and salivary glands. SPECT/CT of mice given 20 MBq of 99mTcO4− was performed. At end of scan, Iomeron was administered intravenously, sublingually, enterally, and intraperitoneally. Four days later, animals were injected with 20 MBq of 99mTcO4− and new scans were obtained. Data are percentage radiotracer taken up by thyroid (A) and salivary glands (B) (n = 2 per condition). ***P < 0.001. %IA = percentage injected activity.
DISCUSSION
Although rare, side effects of the use of ICM, such as thyrotoxicosis, hyperthyroidism, and hypothyroidism, have been reported, both in populations at risk and in some cases in individuals without previous thyroid dysfunction (4,25,26). However, the main side effect observed is the perturbation of iodide uptake by the thyroid. This effect does not appear to affect total intrathyroidal iodine concentration (27). This perturbation has been attributed to the free iodide associated with or released from ICM on administration. We first determined whether free iodide contamination associated with the ICM could mediate this effect. Mass spectrometric analysis was used to quantify the free iodide in Iomeron (25 to 30 μM free iodide) and Visipaque (100 μM free iodide). In addition, competition experiments using NIS-expressing cells demonstrated that Iomeron did not affect iodide uptake. These in vitro results suggest that if free iodide is involved in the perturbation of iodide uptake by the thyroid in vivo, it must be released in the body from the deiodination of ICM in tissues that may store ICM. The possibility of such ICM deiodination has been demonstrated (4,12), and considering the high amount of iodide associated with the organic backbone of the ICM, deiodination may be the source of a delayed release of iodide to the circulation sufficient to perturb thyroid uptake.
Free iodide perturbed uptake of the iodide analog, 99mTcO4−, by both mouse thyroid and mouse salivary glands. Kinetically, for both organs, uptake decreased rapidly (30 min after KI injection in our experiment), and the capacity of the thyroid to take up the radiotracer was restored within 24 h. In comparison, iomeprol affected uptake of 99mTcO4− to mouse thyroid selectively. As expected from the well-known biodistribution of these 2 radiotracers (28), this effect was also observed with 123I. Figure 3 represents 2 scintiscans obtained from human patients. These human data are consistent with the animal results. However, considering the small number of cases, this dataset can be taken as only an indication rather than a formal demonstration of a differential uptake of 99mTcO4− by the thyroid and salivary glands on ICM administration. Nevertheless, overall, our data rule out a direct intervention of free iodide in the perturbation of uptake to the thyroid and suggest a direct and selective effect of iomeprol on the thyroid. Considering that similar data were obtained with Visipaque, it is tempting to hypothesize that ICM, in general, act directly and selectively on the thyroid.
In patients, urinary iodide content has been shown to be elevated for several weeks after ICM injection, and a 4- to-6-wk period is considered necessary for urinary iodide to return to normal levels (10,11,29,30). These observations provide the basis for the advised 4- to 6-wk delay between ICM injection and radioiodine therapy in thyroid cancer patients (7). This implies that urinary iodide has been used as a surrogate marker of the restoration of normal iodide uptake by thyroid tissues. Our results cast serious doubt on the relevance and usefulness of the measurement of urinary iodide concentration in the context of ICM and radioiodine therapy. In our experimental conditions, in mice, the ICM-mediated thyroid stunning lasted for at least 8 d and a recovery was observed by day 12. These data are consistent with a long-lasting effect of a single injection of ICM in humans. The recommended delay between ICM injection and radioiodine therapy is currently between 4 and 8 wk. If the recommended delay between ICM injection and radioiodine therapy were to be reduced or optimized, we advocate that dedicated trials should be performed and based on functional imaging of the thyroid.
The mechanism by which ICM reduces iodide uptake by the thyroid involves a dramatic reduction in the expression of the NIS protein. Four days after ICM injection, the NIS protein was hardly detectable using immunohistochemistry. Western blot analysis confirmed this observation. As expected from the functional imaging data, NIS protein expression was unaffected in the salivary glands. To exert this effect, it is likely that ICM trigger an intracellular molecular mechanism that results in reduced NIS protein expression. ICM have been shown to trigger various intracellular signaling pathways in kidney cells (31–33), which could be involved in the effect observed on the thyroid. The molecular mechanisms involved in the thyroid-specific effect of ICM are currently under investigation in our laboratory.
Although effective and safe overall, the use of KI tablets to protect populations in the event of a nuclear incident is associated with some problematic issues. In cases of prolonged 131I exposure, the U.S. Food and Drug Administration recommends that KI tablets be ingested on a daily basis (34), with potential logistic and compliance problems. In this context, “one-shot” measures that could replace KI tablets or reduce the requirement for a stringent compliance to the daily intake of KI tablets would be welcome. Formulations based on ICM could provide this benefit. Given that the intravenous mode of administration is hardly relevant for an application in radioprotection, the sublingual delivery of ICM is efficient in mice and could be considered in humans. In addition, ICM could be chemically modified to be absorbed by the gut epithelium, leading the way to a compound administered orally. Furthermore, elucidation of the molecular mechanisms involved in the effects of ICM on the thyroid could provide a basis for the selection of new compounds that could be used in radioprotection.
CONCLUSION
One side effect of the use of ICM is alteration of iodide uptake by the thyroid. This effect is thought to be mediated by free iodide associated with or released from ICM. In the present report, we demonstrated that ICM induces thyroid stunning to a greater and longer-lasting degree than the free iodide found in ICM would explain. The effect is due to a specific and dramatic decrease in NIS expression in thyrocytes. These data cast serious doubt on the relevance and usefulness of the measurement of urinary iodide concentration to evaluate the delay between ICM administration and radioiodine therapy of patients with differentiated thyroid carcinoma. Finally, the ability of ICM to perturb iodide uptake in the thyroid on a relatively long-term basis could be exploited in radioprotection.
DISCLOSURE
This work was supported by INSERM, the French National Research Agency (ANR), and a grant from “Plan Cancer 2014-2019” (MTS201403). Equipment for this study was purchased through grants from “la Région Provence Alpes-Côte d’Azur.” A patent on the utilization of ICM in radioprotection is currently being processed. The IRCAN Histology core facility is supported by the University of Nice Sophia-Antipolis and INSERM. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank the radiopharmaceutical team of the Centre Antoine Lacassagne (Nadine Sapin, Guy Martinico, Stéphane Espitallier, and Didier Alberato) for their help with radioisotope production and handling. We thank Colette Ricort for assistance in preparing the manuscript. We thank Catherine Pons in the IRCAN Histology core facility. We thank the IRCAN Animal Core Facility for providing access to their equipment.
Footnotes
Published online Oct. 19, 2017.
- © 2018 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication May 4, 2017.
- Accepted for publication August 1, 2017.