Abstract
In prostate cancer (PC) patients, the differentiation between lung metastases and lesions of different origin, for example, primary lung cancer, is a common clinical question. Herein, we investigated the use of Glu-NH-CO-NH-Lys(Ahx)-HBED-CC (68Ga-PSMA-HBED-CC) for this purpose. Methods: PC patients (n = 1,889) undergoing 68Ga-PSMA PET/CT or PET/MR scans were evaluated retrospectively for suggestive lung lesions. For up to 5 lesions per patient, location, CT diameter, CT morphology, and SUVmax were determined. The standard for classification was either histopathologic evaluation or, in the case of PC metastases, responsivity to antihormone therapy. A comparison of the different classes was executed by Student t test. Prostate-specific antigen and prostate-specific membrane antigen (PSMA) immunohistochemistry were performed if histologic samples were available; 68Ga-PSMA autoradiography was performed on an exemplary case of PET-positive lung cancer. Results: Eighty-nine lesions in 45 patients were identified, of which 76 were classified as PC (39 proven, 37 highly probable), 7 as primary lung cancer, and 2 as activated tuberculosis; 4 lesions remained unclear. The mean SUVmax was 4.4 ± 3.9 for PC metastases and 5.6 ± 1.6 for primary lung cancer (P = 0.408). Additionally, substantial differences in SUVmax intraindividually were detected. The 2 tuberculous lesions showed an SUVmax of 7.8 and 2.5. Using immunohistochemistry, we could demonstrate PSMA expression in the neovasculature of several PSMA PET-positive lung cancers as well as in tuberculous lesions from our histologic database. Conclusion: Quantitative (SUV) analysis of 68Ga-PSMA PET was not able to discriminate reliably between pulmonary metastases and primary lung cancer in PC patients. The reason for the unexpectedly high tracer uptake in non-PC lesions is not completely clear. PSMA expression in neovasculature provides a possible explanation for this finding; however, other contributing factors, such as tracer binding to proteins other than PSMA, cannot be excluded at present.
Radiolabeled prostate-specific membrane antigen (PSMA) ligands are increasingly used in the work-up of patients with prostate cancer (PC). PSMA, or glutamate carboxypeptidase II, is a membrane-bound protein expressed in the prostate and a variety of other tissues (1,2). It shows significantly elevated expression levels in PC compared with benign prostatic cells (3), making it a promising target for molecular imaging. Glu-NH-CO-NH-Lys(Ahx)-HBED-CC (68Ga-PSMA-HBED-CC) is an extracellular PSMA inhibitor combining a urea-based binding motif with an interaction between the HBED-CC chelator and a lipophilic pocket in the extracellular PSMA domain (4). PET with 68Ga-labeled PSMA-HBED-CC has been shown to be of high clinical value for lymph node staging and detection of local recurrence in PC patients, resulting in detection rates of up to 97% depending on prostate-specific antigen (PSA) levels (5,6). Although these excellent results are consistent with a high specificity based on preclinical studies (7), there have also been reports on tracer enhancement in benign lesions and other tumor entities (8,9). This phenomenon has been attributed mainly to PSMA expression in neovasculature and specialized (e.g., endocrine) cells as demonstrated by immunohistochemistry (3).
Visceral metastases are encountered less often than lymph node or bone metastases and occur predominantly in the later course of disease (10) but affect prognosis severely (11) and may lead to changes in the therapeutic regimen. The predominant sites of visceral metastases in a postmortem study were lung (46% of patients with distant metastases), liver (25%), pleura (21%), and adrenals (13%) (12). The differentiation between PC metastases and lesions of different origin using conventional imaging may be challenging and, in many cases, warrants histologic clarification. Imaging options for lung nodules are especially restricted, because only plain x-ray, CT, and 18F-FDG PET are clinically established for this purpose (13)—the last, though, is of limited value in PC (14). Herein, we investigated the value of 68Ga-PSMA PET for the diagnostic work-up of suggestive lung nodules in PC patients and evaluated whether the high specificity of PSMA PET can help in identifying pulmonary PC metastases.
MATERIALS AND METHODS
Patients
From a total number of 1,889 individuals who underwent 68Ga-PSMA-ligand PET/CT or PET/MR for staging or restaging of PC from the institutions’ database (November 2012 to March 2015), patients with suggestive pulmonary nodules in CT were extracted retrospectively. Only intrapulmonary lesions, but no thoracic lymph nodes, were analyzed. Lesions with a maximum diameter of less than 7 mm were excluded to minimize partial-volume effects in PET (15); in addition, lesions had to be either increasing in size compared with earlier CT scans, multiple lesions, or irregularly configured. Stable nodules compared with previous or follow-up CT without specific therapy as well as those with classic signs of benignity (fat or calcification) were excluded.
All patients gave written informed consent for the purpose of anonymized evaluation and publication of their data. The retrospective analysis was approved by the institutional review board of the Technical University of Munich (permit 5665/13).
Classification
Lesions were classified into the following groups: PC all, which was further divided into PC highly probable and PC-proven; lung cancer; other entity; and unclear (Tables 1 and 2). Diagnosis of PC metastases was proven either by histology or, if pulmonary lesions showed clear regression, by antihormone therapy. If these criteria were not fulfilled, the class PC metastases highly probable was chosen for multiple suggestive lung lesions in a patient with other distant metastases, no other known malignancy, and elevated PSA levels. Lung cancer was reserved for lesions with a conclusive histology. The class other entity was chosen for lesions with a conclusive histology or sputum analysis proving a non-PC origin. The remaining suggestive lung lesions were classified as unclear.
Patients (n = 45)
Lesion Characteristics
Synthesis and Application of 68Ga-PSMA-Ligand
Images were obtained with HBED-CC (7) that was labeled with 68Ga3+ (half-life, 67.6 min) from a 68Ge/68Ga radionuclide generator (iThemba Labs) by means of a fully automated module (Scintomics) and good manufacturing practice–grade disposable cassettes and reagent kit (ABX) as described previously (16). The final product was dissolved in isotonic phosphate-buffered saline with subsequent sterile filtration.
The 68Ga-PSMA-ligand complex solution was applied to patients via an intravenous bolus (mean, 151 ± 30 MBq; range, 95–236 MBq). Variation of injected radiotracer activity was caused by the short half-life of 68Ga and variable elution efficiencies obtained during the lifetime of the 68Ge/68Ga radionuclide generator.
Imaging Protocol
PET acquisition was started at a mean time of 54.7 ± 8.5 min after tracer injection (median, 54 min; range, 45–80 min). Forty-one patients underwent 68Ga-PSMA PET/CT on a Biograph mCT scanner (Siemens Medical Solutions), and 4 patients underwent 68Ga-PSMA PET/MR on a Biograph mMR scanner (Siemens Medical Solutions). PET/CT and PET/MR were performed as previously described (5,17). Notably, for the evaluation of lung lesions an inspiratory CT of the thorax was acquired in PET/CT and an axial contrast-enhanced T1-weighted volumetric interpolated breath-hold examination sequence in PET/MR; additional inspiratory CT acquisitions were available for all PET/MR patients. All PET images were acquired in 3-dimensional mode and reconstructed by an attenuation-weighted ordered-subsets expectation maximization algorithm (4 iterations, 8 subsets) followed by a postreconstruction smoothing gaussian filter (5 mm in full width at half maximum).
Image Analysis
For up to 5 suggestive lung lesions per patient, starting from the biggest, location, morphology, CT diameter, and SUVmax in PSMA PET were obtained. 68Ga-PSMA-ligand PET and CT or MR images were interpreted by 1 board-certified nuclear medicine physician and 1 board-certified radiologist, followed by a final consensus interpretation.
Statistical Analysis
For the different classes of lesions as described above, SUVmax was analyzed, and box-and-whisker plots were created. SUVmax of the classes PC all and PC-proven was compared with the class lung cancer using the Student t test. Tests were performed 2-sided, and a level of significance of α = 0.05 was used. Statistical analyses were conducted with Prism 6.1 (GraphPad Software Inc.).
Histology
For histologic evaluation, all specimens from our imaged cohort, as well as 2 exemplary cases of tuberculosis from our histologic database, were fixed in 10% buffered formalin and paraffin-embedded according to standard procedures. Tissue sections (3 μm) were subsequently stained with hematoxylin and eosin and periodic acid-Schiff stain for diagnostic purposes. Immunohistochemistry used anti-PSMA antibodies (clone 3E6 [Dako]; clone 7E11 [kindly provided by Dr. Jan Grimm, Memorial Sloan Kettering Cancer Center]) and PSA antibodies (polyclonal rabbit antihuman PSA antibody; Dako) to label PC cells. An automated immunostaining device (BenchMark XT IHC/ISH; Ventana) was used according to the manufacturer’s protocol (XT ultraView DAB v3) with a citrate buffer (pH 6.0) and DAB (diaminobenzidine) as chromogen as well as hematoxylin for counterstaining.
For autoradiography, the fresh frozen tissue of 1 exemplary case of primary lung cancer, which had shown a clear signal in PSMA PET, was cryosectioned in 15-μm slices, fixed in Delauney’s solution for 1 min, and air-dried. Afterward, the specimens were incubated with 68Ga-PSMA-HBED-CC (30 kBq/mL) in (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer (25 mM, pH 7.4). Exposure was for 4 h on a BAS-IV TR 2025 E Tritium screen (Fujifilm).
RESULTS
Forty-five patients who exhibited suggestive lung lesions were identified. According to the criteria defined above, in 18 patients lesions were classified as PC-proven, in 15 as PC highly probable, and in 8 as other entity (7 cases of lung cancer and 1 case of reactivated tuberculosis). In 3 patients, the origin of the lung lesions remained unclear. There were no cases in which multiple classifications in 1 patient were present. Patient details are listed in Table 1.
The total number of suggestive pulmonary lesions was 89. Detailed lesion characteristics are given in Table 2. Thirty-nine lesions were classified as PC-proven, 37 as PC highly probable, 7 as lung cancer–proven, and 2 as other entity (both resembling activated tuberculous lesions according to sputum analysis). Four lesions remained unclear. The mean SUVmax was 4.3 ± 3.7 for all lesions, 4.4 ± 3.9 for all PC metastases, 5.0 ± 4.4 for histologically proven PC metastases, and 5.6 ± 1.6 for proven primary lung carcinoma. The 2 tuberculous lesions showed an SUVmax of 7.8 and 2.5.
Figures 1 and 2 show imaging examples of patients with pulmonary PC metastasis (PSMA PET/CT) and primary lung cancer (PSMA and 18F-FDG PET/CT). Interestingly, the latter also exhibited 2 mediastinal lymph nodes, which were positive in both PSMA and 18F-FDG PET scans and represented histologically proven lymph node metastases. This represents only an illustrative case for which lymph node uptake was evaluated, and it was not included into the statistical analysis.
Example PSMA PET/CT scan of patient with pulmonary PC metastasis. (A) CT shows irregularly shaped lesion in left upper lobe in PC patient after radical prostatectomy, no known metastases, and PSA of 1.58 ng/mL. Lesion was subsequently biopsied and diagnosed as pulmonary PC metastasis. (B) PSMA PET is positive, with SUVmax of 7.1. (C) PET/CT fusion image.
Example PET/CT scans of patient with primary lung cancer (adenocarcinoma). (A) CT shows spiculated lesion in right upper lobe in PC patient after prostatectomy and suspected local recurrence; PSA was 4.09 ng/mL. (B) PSMA PET exhibits focal tracer enhancement in pulmonary lesion (SUVmax, 5.3) and in 2 mediastinal lymph nodes, which were histologically confirmed as primary lung cancer and associated lymph node metastases. This was the only case for which lymph node uptake was evaluated. (C) 18F-FDG PET as established imaging method of choice for lung cancer shows similar enhancement in primary tumor and 2 mediastinal lymph node metastases.
No significant difference in SUVmax was observed when lung cancer was compared with all PC (P = 0.408; Fig. 3) or when lung cancer was compared with histologically proven PC only (P = 0.740), although an SUVmax of 8 or greater (9/89 lesions) occurred only in PC metastases. An intraindividual comparison in patients with multiple lesions showed large differences in SUV between different nodules in 1 patient (Fig. 4), which cannot be attributed solely to partial-volume effects (e.g., patient 1 exhibited an SUVmax from 3.1 to 20.4 while all lesions were 12–13 mm in diameter). Morphology was not distinctive either because, although all primary lung cancers had an irregular or spiculated appearance, this morphologic feature was also demonstrated in 27 (35.6%) of 76 PC metastases.
Distribution of SUVmax for different lesion classes. No significant difference was shown when comparing SUVmax distributions between groups: PC all (n = 76) consisting of PC-proven and PC highly probable vs. lung cancer–proven (n = 7) (P = 0.408) and PC-proven (n = 39) only vs. lung cancer–proven (P = 0.780).
Intraindividual variability of PSMA uptake in pulmonary PC metastases. Shown is distribution of SUVmax for proven or probable pulmonary PC metastases in patients exhibiting 3 or more lesions.
PSMA immunohistochemistry was evaluated for the primary non–small cell lung cancers in our cohort. We could detect PSMA expression in tumor-associated neovasculature in all specimens (Fig. 5C). PSA immunohistochemistry was also performed but was negative for all samples. Furthermore, we extracted 2 cases of tuberculosis from our histologic database and could also demonstrate PSMA-positive vessels (Fig. 5D). No other cell-specific PSMA antibody binding was detected.
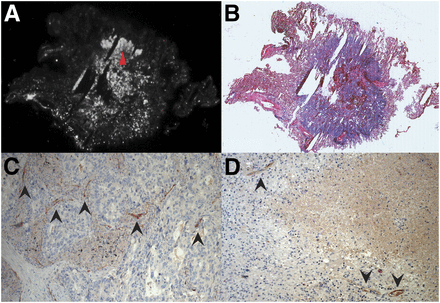
Autoradiography and PSMA immunohistochemistry of exemplary case of primary lung cancer and PSMA immunohistology of tuberculous lesion. (A) PSMA autoradiography of primary lung carcinoma from imaged cohort, which exhibited strong signal in PSMA PET (SUVmax, 5.2). (B) Hematoxylin and eosin stain showing tumor contours. (C) PSMA immunohistochemistry of same tumor, showing detail of tumor region with high cell density and high signal in autoradiography (red arrowhead in A), exhibiting no tumor cells with clear PSMA expression but PSMA in neovasculature (black arrowheads). (D) PSMA immunohistochemistry of case of active tuberculosis from our histologic database, showing PSMA-positive blood vessels (black arrowheads).
Postoperative PSMA autoradiography was established on frozen slices of PC tissue (Fig. 6, unpublished data). This procedure was used for an exemplary PSMA PET-positive lung tumor from the imaged cohort (SUVmax, 5.2) for which fresh frozen tissue was available. The PSA blood level was 0.44 ng/mL; histologic evaluation classified this tumor as a primary adenocarcinoma of the lung. Diffuse PSMA tracer binding was detected in autoradiography, which was apparently not limited to blood vessels (Figs. 6A and 6B).
68Ga-PSMA autoradiography and PSMA immunohistochemistry of prostate carcinoma PC samples were used to establish autoradiography and immunohistology protocols. (A) PSMA autoradiography. (B) Hematoxylin and eosin stain. (C) Immunohistochemistry with anti-PSMA antibody 7E11 (aliquot kindly provided by Dr. Jan Grimm, Memorial Sloan Kettering Cancer Center).
DISCUSSION
In this study, we retrospectively investigated the potential value of 68Ga-PSMA-HBED-CC PET/CT for the differential diagnosis of suggestive lung lesions in PC patients. The differentiation of potentially malignant pulmonary lesions is of considerable clinical importance; the occurrence of distant metastases adversely affects the prognosis of PC patients and will often lead to modifications in the treatment regime, mostly systemic antihormone or chemotherapy. On the other hand, primary lung carcinoma, the second most common malignant tumor in men, is potentially curable by resection. Conventional imaging options for thoracic lesions are restricted, with only plain x-ray and CT being used in common clinical practice. The reported high specificity of PSMA PET for lymph node staging in PC patients raises expectations of a similar usefulness for the diagnosis of pulmonary metastases.
Our results do not support these expectations. Conversely, we found an unanticipated high PSMA PET uptake in primary lung tumors. Although in our cohort only PC metastases showed an SUVmax of greater than 8, a clear differentiation between PC metastases and primary lung cancer, for example, by an SUVmax cutoff, is not possible according to our data. The specificity of PSMA PET is further challenged by pronounced tracer uptake in 2 lesions resembling activated tuberculosis. Sensitivity was not as high as expected either, because several proven pulmonary PC metastases showed only faint uptake in PSMA PET, which might be explained in part by response to previous therapies. However, as a consequence, all suggestive lesions, whether exhibiting uptake in PSMA PET or not, should either be followed up by CT or PET/CT or be clarified by biopsy.
We should underline here that it was not the purpose of this study to differentiate between benign and malignant lesions by PSMA PET. Considering the inclusion criteria, it is evident that many common benign lesions, such as postinflammatory scars or granulomas, were excluded from the study. In fact, besides the 2 tuberculous lesions, there were no cases of proven benign nodules in our cohort, though some of the unclear lesions may well have been nonmalignant.
The reason for PSMA-HBED-CC tracer enhancement in non-PC lesions is not completely clear: free 68Ga binding, for example, to CD71, was considered but seems rather unlikely because of the high radiochemical purity of the injected tracer. Using immunohistochemistry, we could demonstrate PSMA expression in the neovasculature of PSMA PET-positive lung carcinomas, a phenomenon that has been reported before for different tumors (18). Furthermore, and rather unexpectedly, we could also show PSMA immunostaining in the neovasculature of 2 exemplary cases of tuberculosis from our histologic database; reports on PSMA expression in nonneoplastic regenerative tissue can as well be found in the literature (19). The low density of PSMA-positive vessels, however, casts some doubt on the hypothesis that this phenomenon alone is responsible for the considerable amount of PSMA tracer uptake seen in these lesions. The doubts are substantiated by the demonstration of diffuse tracer binding in an exemplary autoradiography of a primary lung tumor from our cohort, whereas PSMA expression in lung tumor cells, though reported previously (20), could not be verified. This result raises the question whether binding to a substance other than PSMA might be a possible contributing factor. Natural candidate proteins would include PSMA homologs, such as glutamate carboxypeptidase III (21,22) or N-acetylated α-linked acidic dipeptidaselike protein (23). Further insight could be gained by performing tracer uptake assays on tumor cell lines, therefore excluding the effect of tracer binding to neovasculature.
Our study is limited by its retrospective nature and the relatively low number of proven non-PC lesions in the investigated cohort. In addition, we cannot exclude that, in some cases, biopsies of unclear lesions were sought because of high uptake in PET imaging, which might have induced further bias. However, we think that the most important implication—the lack of discriminative power of PSMA-HBED-CC PET imaging regarding the underlying histology of suggestive lung lesions—is not critically affected by these limitations.
CONCLUSION
Quantitative analysis of 68Ga-PSMA-HBED PET was not able to discriminate reliably between pulmonary lesions of different origin in PC patients. Primary lung tumors showed unexpectedly high tracer uptake similar to PC metastases. PSMA expression in neovasculature provides a possible explanation for these findings; however, other contributing factors, such as tracer binding to proteins other than PSMA, cannot be excluded at present.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This study was supported by funding from the Faculty of Medicine of the Technical University of Munich (grant KKF B11-14) and the European Union Seventh Framework Program (FP7) under grant agreement no. 294582 ERC Grant MUMI. The development of 68Ga-PSMA synthesis was supported by SFB 824 (Project Z1) from the Deutsche Forschungsgemeinschaft, Bonn, Germany. The research leading to these results has received funding from the European Union Seventh Framework Program (FP7) under grant agreement no. 256984 EndoTOFPET. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Nov. 19, 2015.
- © 2016 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication July 24, 2015.
- Accepted for publication October 28, 2015.