Abstract
l-3-18F-α-methyl tyrosine (18F-FAMT) has been developed as a PET radiotracer for tumor imaging. Clinical studies have demonstrated the usefulness of 18F-FAMT PET for the prediction of prognosis and the differentiation of malignant tumors and benign lesions. 18F-FAMT exhibits higher cancer specificity in peripheral organs than other amino acid PET tracers and 18F-FDG. The accumulation of 18F-FAMT is strongly correlated with the expression of L-type amino acid transporter 1 (LAT1), an isoform of system L highly upregulated in cancers. In this study, we examined the interaction of 3-fluoro-l-α-methyl-tyrosine (FAMT) with amino acid transporters to assess the mechanisms of 18F-FAMT uptake in PET. Methods: We applied in vitro assays using established mammalian cell lines stably expressing LAT1 or a non–cancer-type system L isoform LAT2. The inhibitory effect on l-14C-leucine uptake and the induction effect on efflux of preloaded l-14C-leucine were examined for FAMT and other amino acid tracers. FAMT transport was compared among cell lines with varied LAT1 expression level. Results: FAMT prominently inhibited LAT1-mediated l-14C-leucine uptake in a competitive manner but had less of an effect on LAT2. In the efflux experiments, FAMT induced the efflux of preloaded l-14C-leucine through LAT1, indicating that FAMT is transported by LAT1 and not by LAT2. Among amino acid–related compounds examined in this study, including those used for PET tracers, the compounds with an α-methyl group such as FAMT, 2-fluoro-l-α-methyl-tyrosine, 3-iodo-l-α-methyl-tyrosine, and l-α-methyl-tyrosine were well transported by LAT1 but not by LAT2. However, l-methionine, l-tyrosine, 3-fluoro-l-tyrosine, 2-fluoro-l-tyrosine, and O-(2-fluoroethyl)-l-tyrosine were transported by both LAT1 and LAT2, suggesting that the α-methyl moiety is responsible for the LAT1 selectivity of FAMT. FAMT transport rate and LAT1 protein level were well correlated, supporting the importance of LAT1 for the cellular uptake of FAMT. Conclusion: Distinct from other amino acid PET tracers, because of its α-methyl moiety, FAMT is selective to LAT1 and not transported by LAT2. This property of FAMT is proposed to contribute to highly tumor-specific accumulation of 18F-FAMT in PET.
The most widely used PET radiotracer for tumor imaging at the moment is 18F-FDG, a glucose analog that accumulates in tumor cells via glucose transporters on the plasma membrane (1). However, the well-recognized limitation of 18F-FDG PET is high uptake in inflammatory lesions and granulation tissues and high physiologic background uptake in some normal tissues, giving rise to false-positive diagnostic findings (2). Amino acid tracers that in general exhibit a more tumor-selective nature have been considered to overcome such disadvantages of 18F-FDG PET (3). l-methyl-11C-methionine (11C-MET), the most commonly used amino acid PET tracer, and the well-studied O-(2-18F-fluoroethyl)-l-tyrosine (18F-FET), for example, show lower uptake in normal tissues and inflammatory lesions than 18F-FDG (3). However, most of the amino acid PET tracers, including 11C-MET and 18F-FET, still suffer from various levels of physiologic background uptake (4,5). The nonnatural amino acid analogs that are highly selective to cancers and do not accumulate in normal tissues and noncancer lesions would be beneficial for high-specificity cancer diagnosis with PET.
We developed l-3-18F-α-methyl tyrosine (18F-FAMT) as an amino acid PET tracer and showed that 18F-FAMT accumulates specifically in tumors, confirming its usefulness in the detection of cancers (6–11). Recent clinical studies demonstrated that 18F-FAMT PET is useful for the prediction of prognosis in non–small cell lung cancer and the differentiation of malignant tumors from benign lesions (9–11). In addition, physiologic background in 18F-FAMT PET is low, except in the kidneys and urinary bladder, which are in the excretion path of 18F-FAMT (8). These findings let us speculate that 18F-FAMT accumulates in cancer cells by the amino acid transporters specifically expressed in cancers and not by transporters of noncancer cells, whereas other amino acid tracers are taken up by the transporters of both cancer and noncancer cells. In fact, the accumulation of 18F-FAMT in non–small cell lung cancer is strongly correlated with the expression of L-type amino acid transporter 1 (LAT1), an isoform of system L highly upregulated in many cancers (9,11).
System L is a Na+-independent amino acid transport agency mediating the cellular uptake of large neutral amino acids. So far, 4 isoforms of system L transporters have been identified: LAT1, LAT2, LAT3, and LAT4 (12–15). LAT1 and LAT2 belong to the solute carrier 7 (SLC7) family and form heterodimers with a single-membrane–spanning glycoprotein, the heavy chain of 4F2 cell surface antigen (4F2hc/CD98hc/SLC3A2), to be functional on the plasma membrane (16). LAT3 and LAT4 belong to the SLC43 family and do not require 4F2hc (14,15). Among them, LAT1 is widely expressed in primary human tumors of various tissue origins, such as the brain, colon, lung, liver, thymus, ovary, and skin and cancer cell lines, in which it plays essential roles in the growth and survival of cancer cells (12,17–20). LAT1 is upregulated in malignant tumors, and its expression is associated with tumor proliferation, angiogenesis, and poor prognosis (9,11,19,20). In contrast, LAT2 expression is not detected in cancer cells but is widely found in normal tissues, including epithelia of the small intestine and kidney, and thus is regarded as a non–cancer cell type transporter (13,16). We previously found that the expression of LAT1 is an independent and significant factor for predicting a poor prognosis in patients with non–small cell lung cancer, suggesting that the prognostic significance of 18F-FAMT uptake is closely related to LAT1 expression (10,19,20). Although it has been proposed that 18F-FAMT is taken up into cancer cells by LAT1, there is no report that demonstrates the direct transport of FAMT by LAT1 and its kinetic properties. In this study, we examined the interaction of FAMT with the amino acid transporters to assess its mechanisms of uptake in PET.
MATERIALS AND METHODS
Materials
3-fluoro-l-α-methyl-tyrosine (FAMT) and 2-fluoro-l-α-methyl-tyrosine (2-FAMT) were synthesized with chiral phase-transfer alkylation (21) and electrophilic fluorination (6) by Nard Institute, Ltd. FAMT and 2-FAMT were identified by the analysis of 1H-nuclear magnetic resonance (NMR) (ECX-400P; Jeol) and high-performance liquid chromatography (LC-2010A HT; Shimadzu) as follows: FAMT: 1H-NMR (D2O/DCl, 400 MHz): d 1.39 (3H, s), 2.76 (1H, d, J = 14.0 Hz), 3.08 (1H, d, J = 14.0 Hz), and 6.76–6.90 (3H, m); and 2-FAMT: 1H-NMR (D2O/DCl, 400 MHz): d 1.36 (3H, s), 2.94 (2H, s), 6.50–6.55 (2H, m), and 6.96–7.00 (1H, m). The purities of the compounds determined on high-performance liquid chromatography were 99% and 99% enantiomeric excess for both FAMT and 2-FAMT. l-14C-leucine (9.66 GBq/mmol) was from Moravek Biochemicals. Standard amino acids and l-α-methyl-tyrosine (AMT) were from Sigma-Aldrich. 2-Fluoro-l-tyrosine (2FT), O-(2-fluoroethyl)-l-tyrosine (FET), and 3-iodo-l-α-methyl-tyrosine (IMT) were from Advance Biochemical Compounds GmbH. 3-Fluoro-l-tyrosine (3FT) was from Tokyo Chemical Industry. Unless specially denoted, other chemicals were purchased from Wako Pure Chemical Industries. The chemical structures of compounds used in this study are presented in Figure 1.
Chemical structures of compounds used in this study.
Cell Cultures
The mouse renal proximal tubule cell line S2 stably expressing human LAT1 (S2-LAT1) or human LAT2 (S2-LAT2) was used in the present study (22). Using LAT1-specific high-affinity inhibitor KYT-0353 (23) and nonselective system L inhibitor 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid inhibiting both LAT1 and LAT2 (16), we confirmed that l-leucine transport in S2-LAT1 and S2-LAT2 cells is mediated almost exclusively by human LAT1 and LAT2, respectively (data not shown). S2-LAT1 and S2-LAT2 cells were maintained as described previously (22) and used between the passages of 5 and 15, during which period no remarkable change was observed in the level of LAT1 and LAT2 expression determined by Western blot and in the transport activity evaluated by l-14C-leucine uptake. Human cell lines HEK 293 (embryonic kidney cell line), T24 (urinary bladder carcinoma), MIA PaCa-2 (pancreatic carcinoma), DLD-1 (colorectal adenocarcinoma), HeLa S3 (cervical adenocarcinoma), HEp-2 (HeLa subline), and H520 (non–small cell lung carcinoma cell line) were obtained from American Type Culture Collection. The cells were maintained by following the instructions from American Type Culture Collection.
Uptake Measurements and Inhibition Experiments
Uptake measurement was performed as described previously (22). The uptake of l-14C-leucine by the cells was measured for 1 min in Na+-free Hanks balanced salt solution ([HBSS]: 125 mM choline chloride, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1.3 mM CaCl2, 5.6 mM d-glucose, and 25 mM N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid], pH 7.4) containing l-14C-leucine (5.0 MBq/mmol) at the concentration indicated in each experiment. For inhibition experiments, the uptake of l-14C-leucine (1 μM) was measured for 1 min with or without nonradiolabeled test compounds at the indicated concentration. After uptake was terminated, the cells were lysed and the radioactivity was measured by a β-scintillation counter (LSC-3100; Aloka). An aliquot of the lysate was used to determine the protein concentration by bicinchoninic acid assay (Thermo Fisher Scientific).
The half maximal inhibitory effect (IC50) of FAMT and 2-FAMT was determined by experiments in which the uptake of l-14C-leucine (1 μM) was measured for 1 min in the presence of FAMT or 2-FAMT at concentrations of 1, 3, 10, 30, 100, 300, and 1,000 μM (24). The data were fitted to inhibition curves by nonlinear regression (4-parameter Hill function, SigmaPlot 11; Systat Software, Inc.).
The kinetic parameters of uptake inhibition by FAMT were determined on the uptake of l-14C-leucine for 1 min at concentrations of 1, 2, 5, 10, 20, 50, and 100 μM (24). The uptake values in the presence or absence of 50 and 100 μM FAMT were plotted against l-14C-leucine concentration and fitted to a Michaelis–Menten curve. The Michaelis constant (Km) and the maximum velocity values (Vmax) were determined by nonlinear regression (4-parameter Hill function). To obtain the inhibition constant (Ki), the rates of uptake of l-14C-leucine were measured in the presence or absence of 50 μM FAMT. Double reciprocal plots were drawn using the enzyme kinetics module of SigmaPlot 11. The Ki value was calculated by the following equation when competitive inhibition was observed: Ki = concentration of FAMT/([Km of l-14C-leucine with FAMT/Km of l-14C-leucine without FAMT] – 1) (25).
Efflux Measurements
Efflux measurements were performed as described previously (24). The cells were preloaded with 1 μM l-14C-leucine (12.6 MBq/mmol) in Na+-free HBSS for 10 min. The cells were then washed, and the efflux was initiated by incubating the cells for 1 min in Na+-free HBSS medium with or without 40 μM nonradiolabeled test compounds, unless otherwise indicated. After incubation, the medium was collected. The radioactivity in the medium and the remaining radioactivity in cells were counted.
The kinetic parameters of FAMT-induced l-14C-leucine efflux were determined on l-14C-leucine efflux induced by extracellularly applied FAMT at concentrations of 1, 3, 10, 30, 50, 100, 300, and 1,000 μM for 1 min (24). To obtain FAMT-induced l-14C-leucine efflux, the values for l-14C-leucine efflux in the presence of extracellular FAMT were subtracted from those in the absence of FAMT and expressed as the percentage of total radioactivity. The FAMT-induced l-14C-leucine efflux was plotted against FAMT concentration and fitted to a Michaelis–Menten curve. Km and Vmax were determined using an Eadie–Hofstee plot.
Western Blot Analysis
The human cell lines were cultured for 2 d. The cells were lysed in buffer containing 50 mM Tris-Cl (pH 7.4), 120 mM NaCl, 20 mM NaF, 1 mM ethylenediaminetetraacetic acid, 6 mM ethylene glycol tetraacetic acid, 20 mM β-glycerophosphate, 1 mM dithiothreitol, 0.5 mM phenylmethanesulfonyl fluoride, 1 mM Na3VO4, 1% NP-40, and ethylenediaminetetraacetic acid–free protease inhibitor cocktail (Roche Diagnostics GmbH). The Western blot was conducted as described previously (22). Antihuman LAT1 polyclonal antibody (Trans Genic Inc.) and antihuman β-actin monoclonal antibody (Sigma Aldrich) were used at 1:10,000 dilution.
Statistical Analysis
All experiments were performed in 6–8 replications. The data are expressed as mean ± SEM. Statistical differences were determined using the unpaired Student t test. Differences were considered significant at a P value of less than 0.05.
RESULTS
Inhibition of l-14C-Leucine Uptake by FAMT in S2-LAT1 and S2-LAT2 Cells
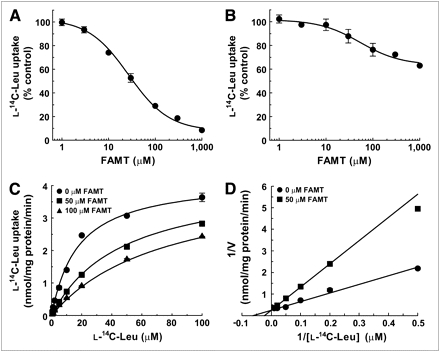
The S2-LAT1 and S2-LAT2 cells were used to study the interaction of FAMT with human LAT1 and LAT2, respectively. The inhibition experiments were first performed to examine whether FAMT interacts with LAT1 and LAT2. FAMT prominently inhibited l-leucine uptake mediated by LAT1 but not by LAT2. As shown in Figure 2A, FAMT inhibited the uptake of l-14C-leucine in a concentration-dependent manner with the IC50 of 26.9 ± 1.1 μM in S2-LAT1 cells. In S2-LAT2 cells, in contrast, FAMT showed less inhibitory effect on the uptake of l-14C-leucine (Fig. 2B). At 1,000 μM, FAMT inhibited l-14C-leucine uptake by 37% in S2-LAT2 cells.
Inhibitory effects of FAMT on l-14C-leucine uptake in S2-LAT1 and S2-LAT2 cells. (A and B) Concentration-dependent inhibition of l-14C-leucine uptake by FAMT in S2-LAT1 cells (A) and S2-LAT2 cells (B). Uptake of 1 μM l-14C-leucine was measured in presence of varied concentrations of FAMT. Uptake values were fitted to inhibition curves. IC50 of FAMT on LAT1-mediated l-14C-leucine uptake was 26.9 μM. (C and D) Kinetics of inhibition of LAT1-mediated l-14C-leucine uptake by FAMT. Uptake of l-14C-leucine (1–100 μM) was measured in absence or presence of FAMT, and plotted against l-14C-leucine concentration to fit to Michaelis–Menten curves (C). Double reciprocal plot analysis was performed on inhibitory effect of 50 μM FAMT (D). Two lines fit to competitive inhibition with FAMT’s Ki value of 29.5 μM. Leu = leucine.
To determine whether the inhibition of LAT1 by FAMT is competitive, the effect of FAMT on the Km and Vmax of LAT1-mediated l-leucine uptake was examined. The uptake of l-14C-leucine was measured at varied concentrations (1–100 μM) in the presence or absence of FAMT. As shown in Figure 2C, l-14C-leucine uptake in the presence of 50 and 100 μM FAMT fitted to a Michaelis–Menten curve, with a Km and Vmax of 45.6 ± 1.6 μM and 4.1 ± 0.1 nmol/mg of protein/min, respectively, for 50 μM FAMT and 72.4 ± 6.7 μM and 4.4 ± 0.2 nmol/mg of protein/min, respectively, for 100 μM FAMT. Km and Vmax in the absence of FAMT were 15.5 ± 1.8 μM and 3.9 ± 0.2 nmol/mg of protein/min, respectively. These findings indicate that Km is increased by FAMT, with Vmax remaining unchanged. Double reciprocal plots confirmed that FAMT (50 μM) altered Km without changing Vmax (Fig. 2D), consistent with the competitive inhibition. Ki value was calculated to be 29.5 ± 3.6 μM. Inhibition kinetics were not obtained for LAT2 because of the low level of inhibition.
l-14C-Leucine Efflux Induced by FAMT
FAMT’s competitive inhibition of LAT1-mediated l-14C-leucine transport suggests that FAMT binds to the substrate binding site of LAT1. To determine whether FAMT is transported as a substrate of LAT1 after binding to the substrate binding site or is just a nontransportable inhibitor, we took advantage of the exchanger property of LAT1 (16,24,25). l-14C-leucine was preloaded to S2-LAT1 cells, and the efflux of preloaded l-14C-leucine from the cells induced by extracellularly applied FAMT was measured to determine whether FAMT is transported by LAT1 as its substrate. As shown in Figure 3A, extracellularly applied FAMT induced l-14C-leucine efflux from S2-LAT1 cells more significantly than that measured in the absence of extracellular FAMT. The level of efflux induced by FAMT was similar to that induced by l-tyrosine, a well-characterized substrate of LAT1, suggesting that FAMT is transported by LAT1 in a manner similar to l-tyrosine. In contrast, the efflux of preloaded l-14C-leucine from S2-LAT2 cells induced by FAMT was much less than that induced by l-tyrosine, which is a well-known substrate of LAT2 (Fig. 3B) (16,26).
Efflux of l-14C-leucine induced by FAMT in S2-LAT1 and S2-LAT2 cells. (A and B) Efflux of preloaded l-14C-leucine from S2-LAT1 cells (A) and S2-LAT2 cells (B) in absence ((−)) or presence of 40 μM l-tyrosine and FAMT. (C) Concentration dependence of effect of FAMT on induction of l-14C-leucine efflux from S2-LAT1 cells. Efflux of preloaded l-14C-leucine from S2-LAT1 cells was measured for 1 min in presence of extracellularly applied FAMT. Radioactivity of l-14C-leucine released from cells in presence of FAMT was subtracted from that in absence of FAMT ((−) in A). Efflux values were fit to Michaelis–Menten curve. Inset shows Eadie–Hofstee plot of l-14C-leucine efflux, which was used to determine kinetic parameters. Apparent Km of 27.5 μM and Vmax of 27.7% radioactivity/min were obtained. *P < 0.05. **P < 0.01. Leu = leucine; NS = not significant.
The kinetic property of LAT1-mediated FAMT transport was then examined by analyzing the dependence of the efflux of preloaded l-14C-leucine on the concentration of extracellularly applied FAMT (1–1,000 μM). The result showed that FAMT induced the efflux of preloaded l-14C-leucine in a concentration-dependent manner (Fig. 3C). The efflux values became saturated at a higher concentration (300–1,000 μM) and fitted to a Michaelis–Menten curve (Fig. 3C). Km was 27.5 ± 0.9 μM, and the maximum l-14C-leucine efflux rate (Vmax) induced by FAMT was 27.7% ± 0.4% radioactivity/min. Kinetic parameters of FAMT-induced l-14C-leucine efflux were not obtained for LAT2 because of the low level of efflux.
Comparison of FAMT with Structurally Related Compounds and Other Amino Acid Tracers
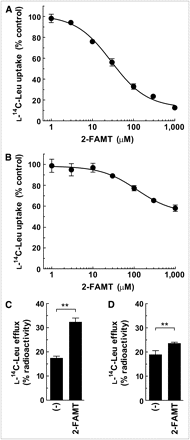
To reveal the effect of fluorine moiety position on the interaction with LAT1 and LAT2, the inhibitory effect and efflux induction were examined on 2-FAMT, which has a fluorine at the 2 position in the aromatic ring (Fig. 1). As shown in Figures 4A and 4B, 2-FAMT inhibited the uptake of l-14C-leucine in a concentration-dependent manner with an IC50 of 28.8 ± 1.2 μM in S2-LAT1 cells, whereas 2-FAMT showed less inhibitory effect in S2-LAT2 cells. At 1,000 μM, 2-FAMT inhibited l-14C-leucine uptake by 42% in S2-LAT2 cells. In the efflux experiments using S2-LAT1 and S2-LAT2 cells preloaded with l-14C-leucine, extracellularly applied 2-FAMT induced a substantial level of l-14C-leucine efflux from S2-LAT1 cells, whereas less l-14C-leucine efflux was induced by 2-FAMT in S2-LAT2 cells (Figs. 4C and 4D).
Interaction of 2-FAMT with LAT1 and LAT2. (A and B) Concentration-dependent inhibition of l-14C-leucine uptake by 2-FAMT in S2-LAT1 cells (A) and S2-LAT2 cells (B). Uptake of 1 μM l-14C-leucine was measured in presence of varied concentrations of 2-FAMT. Uptake values were fitted by nonlinear regression to inhibition curve. IC50 of 2-FAMT on LAT1-mediated l-14C-leucine uptake was 28.8 μM. (C and D) Efflux of preloaded l-14C-leucine from S2-LAT1 cells (C) and S2-LAT2 cells (D) in absence ((−)) or presence of 40 μM 2-FAMT. **P < 0.01. Leu = leucine.
Tyrosine-related compounds structurally related to FAMT and l-methionine were compared with FAMT in terms of the interaction with LAT1 and LAT2. As shown in Figure 5, 200 μM l-tyrosine, 3FT, 2FT, FET, and l-methionine strongly inhibited the uptake of l-14C-leucine in both S2-LAT1 and S2-LAT2 cells in a similar manner. In contrast, FAMT, 2-FAMT, IMT, and AMT showed a strong inhibitory effect on LAT1 but less effect on LAT2.
Inhibitory effects of tyrosine-related compounds and l-methionine on l-14C-leucine uptake in S2-LAT1 and S2-LAT2 cells. Uptake of 1 μM l-14C-leucine was measured for 1 min in absence ((−)) or presence of 200 μM tyrosine-related compounds and l-methionine in S2-LAT1 and S2-LAT2 cells. Uptake values were expressed as percentage of control l-14C-leucine uptake measured in absence of inhibitors. Leu = leucine.
In the efflux experiments, the transport of tyrosine-related compounds and l-methionine by LAT1 and LAT2 was compared with that of FAMT. As shown in Figure 6, l-tyrosine, FAMT, 2-FAMT, IMT, AMT, 3FT, 2FT, FET, and l-methionine induced efflux of preloaded l-14C-leucine through LAT1 at similar levels, whereas the efflux of l-14C-leucine mediated by LAT2 was much less for FAMT, 2-FAMT, IMT, and AMT, compared with l-tyrosine, 3FT, 2FT, FET, and l-methionine.
Efflux of l-14C-leucine induced by tyrosine-related compounds and l-methionine in S2 LAT1 and S2-LAT2 cells. Efflux of preloaded l-14C-leucine from S2-LAT1 and S2-LAT2 cells was measured for 1 min in absence or presence of extracellularly applied indicated compounds (40 μM). Radioactivity released from cells in presence of test compounds was subtracted from radioactivity in their absence. Leu = leucine.
Correlation of FAMT Transport and LAT1 Expression
To confirm the role of LAT1 in FAMT uptake, FAMT transport rate and LAT1 expression level were compared in human cell lines. The FAMT transport rate was measured as the rate of efflux of preloaded l-14C-leucine induced by 40 μM FAMT, and LAT1 protein expression level was evaluated by Western blot. As shown in Figure 7, among human cell lines examined in this study, HEK 293 cells exhibited the lowest LAT1 expression level and the lowest FAMT transport rate. HeLa S3, HEp-2, and H520 cells showed a high level of LAT1 expression and a high FAMT transport rate. T24, MIA PaCa-2, and DLD-1 were in the medium range of LAT1 expression and FAMT transport.
Comparison of FAMT transport rate and LAT1 expression level in human cell lines. FAMT transport rate (top) and LAT1 expression level (bottom) were determined in human cell lines HEK 293, T24, MIA PaCa-2, DLD-1, HeLa S3, HEp-2, and H520. FAMT transport rate was evaluated by measuring efflux of preloaded l-14C-leucine (1 min) induced by 40 μM FAMT. Radioactivity of l-14C-leucine released from cells in presence of FAMT was subtracted from radioactivity in absence of FAMT. Western blot analysis of LAT1 and β-actin were conducted on total cell lysate of indicated human cell lines. Leu = leucine.
DISCUSSION
In this study, using cell lines stably expressing human LAT1 (S2-LAT1 cells) or human LAT2 (S2-LAT2 cells), we investigated the mechanism of FAMT accumulation. FAMT prominently inhibited LAT1-mediated l-14C-leucine uptake in a competitive manner (Fig. 2) and stimulated the LAT1-mediated efflux of preloaded l-14C-leucine (Figs. 3A and 3C), indicating that FAMT binds to the substrate-binding site of LAT1 and is transported by LAT1 as its substrate. The affinity of FAMT evaluated on the basis of Ki for inhibiting l-14C-leucine uptake and Km for inducing l-14C-leucine efflux is similar to that of known LAT1 substrates such as l-tyrosine, l-leucine, and l-methionine (12,25), suggesting that FAMT is transported by LAT1 as efficiently as endogenous substrates. In contrast, FAMT weakly inhibited LAT2-mediated l-14C-leucine uptake (Fig. 2B) and only slightly induced LAT2-mediated l-14C-leucine efflux (Fig. 3B), indicating that FAMT is hardly transported by LAT2. Therefore, FAMT is able to discriminate 2 closely related system L transporters, LAT1 and LAT2, and is selectively transported by LAT1.
FAMT is an l-tyrosine derivative modified by adding a methyl group at the α-carbon and a fluorine moiety at the 3 position of the aromatic ring (Fig. 1) (6). Although l-tyrosine is recognized by both LAT1 and LAT2 as a substrate, FAMT is selective to LAT1 (Figs. 2 and 3). 2-FAMT, which has a fluorine at the 2 position, showed properties similar to FAMT in the effect on LAT1 and LAT2 in terms of inhibition of l-14C-leucine uptake and induction of l-14C-leucine efflux (Fig. 4). 3FT and 2FT that had a fluorine moiety but lacked an α-methyl group (Fig. 1) were transported by both LAT1 and LAT2 in a manner similar to l-tyrosine (Figs. 5 and 6). AMT lacking a fluorine was still LAT1-selective (Figs. 5 and 6). Thus, the presence of a fluorine moiety and its position in the aromatic ring do not affect the interaction with LAT1 and LAT2. In contrast, the presence of the α-methyl group is critical for LAT1 selectivity. IMT and AMT, as well as FAMT and 2-FAMT—which have an α-methyl group (Fig. 1)—were well transported by LAT1, whereas they interacted less with LAT2 (Figs. 5 and 6). Therefore, the α-methyl group but not fluorine is the key moiety for FAMT to be LAT1-selective. Consistent with this, we previously showed that α-methyl phenylalanine and α-methyl 3,4-dihydroxyphenylalanine and AMT inhibited l-14C-leucine uptake mediated by LAT1, but they had less of an effect on LAT2 (22,24,25). Moreover, in the amphibian oocyte expression system, we observed that l-3-125I-α-methyl tyrosine (125I-IMT) is transported by LAT1 but not by LAT2 (27).
Besides LAT1 and LAT2, 2 additional system L transporters, LAT3 and LAT4, were identified in the transporter family distinct from that of LAT1 and LAT2 (14,15). There are some reports indicating that LAT3 and LAT4 are not only expressed in normal tissues but also upregulated in some cancers (14,28). LAT3 and LAT4 mainly transport l-phenylalanine, l-methionine, and branched chain amino acids, whereas they interact less with l-tyrosine. It was also shown that AMT does not inhibit LAT4-mediated transport (15). We furthermore confirmed that AMT does not inhibit LAT3-mediated l-leucine transport using a Xenopus oocyte expression system (data not shown). The fact that l-tyrosine and AMT interact less with LAT3 and LAT4 suggests that FAMT would not be a substrate of LAT3 and LAT4. Thus, FAMT is specific to LAT1 among system L transporters.
Other candidate transporters that might interact with aromatic amino acid PET tracers are system T, B0, and B0,+ (Table 1). We showed, in a previous study, that system T transporter TAT1 does not transport α-methyl amino acids (29), suggesting that FAMT is not transported by system T because of its α-methyl moiety. System B0 and B0,+ transporters were previously suggested to somewhat transport a SPECT tracer, l-3-123I-α-methyl tyrosine (123I-IMT) (30,31). System B0, a Na+-dependent broad-scope neutral amino acid transporter, is present on the apical membrane of renal proximal tubules and small intestine and responsible for the absorption of neutral amino acids from the lumen (26). Thus, system B0 was proposed to contribute to the renal accumulation of 123I-IMT and 125I-IMT (31). Although direct interaction of FAMT and IMT with system B0 transporters was not examined, it is possible that the accumulation of 18F-FAMT in kidney in PET is also due to the reabsorption of 18F-FAMT from tubular lumen to tubular epithelial cells via system B0 transporters. System B0,+ transporter ATB0,+, which mediates Na+-dependent broad-scope transport of neutral and basic amino acids, was reported to be expressed mainly in the lung, trachea, and salivary gland and at a lower level in the mammary gland, stomach, and pituitary gland in humans (32). However, the significance of system B0,+ in 123I-IMT SPECT is not considered to be high because of the low background accumulation of 123I-IMT in tissues expressing ATB0,+ and because of the low contribution of system B0,+ to total 123I-IMT uptake in tumor cells. B0,+-mediated uptake accounts for approximately 15% of total 123I-IMT uptake in glioma cells, whereas approximately 70% of the uptake is due to system L (30). Similar to 123I-IMT SPECT, the contribution of system B0,+ to 18F-FAMT PET would not be high, considering the low accumulation of 18F-FAMT in tissues expressing systems B0,+ (8). It was previously reported that the uptake of 3-O-methyl-6-18F-fluoro-l-dopa was not correlated with LAT1 messenger RNA level (28). In the present study, we compared FAMT transport rate with LAT1 protein level, which reflects more directly transport functions, and we obtained a good correlation between them (Fig. 7). This observation at least supports the importance of LAT1 for the cellular uptake of FAMT, although the idea that FAMT is specific to LAT1 among transporters of plasma membrane needs to be confirmed experimentally.
Amino Acid Transporters for Uptake of Amino Acid PET/SPECT Tracers
Among amino acid analogs used for PET tracers tested in this study, only FAMT prefers LAT1 to LAT2. l-methionine, l-tyrosine, FET, 3FT, and 2FT are all transported by both LAT1 and LAT2 (Figs. 5 and 6) (33). The uptake of 11C-MET is recognized in the pancreas, liver, and inflammatory lesions (4,34,35). Relatively strong signals of 11C-MET in these tissues may be due to protein incorporation, because pancreatic accumulation is also found for 11C-tyrosine and 18F-2FT, which are incorporated into proteins (35–37). Another possible explanation for the uptake of 11C-MET in nontumor tissues and inflammatory lesions is the multiple transport systems involved in l-methionine transport. l-methionine is a substrate of systems L, A, ASC, y+L, b0,+, B0, and B0,+ (Table 1) (16,26,38). 18F-FET and 18F-FAMT show lower physiologic backgrounds and accumulate more selectively to malignant tumors, probably because aromatic amino acids are transported by more limited transport systems than l-methionine. 18F-FET, however, accumulates in reactive astrocytes around brain abscesses and shows physiologic background uptake in skeletal muscle (5,35,39). Some investigators have proposed that 18F-FET is taken up by skeletal muscle via LAT2 expressed in the muscle (5,40). Consistent with this proposal, 18F-FAMT and 123I-IMT, both specific to LAT1 and not transported by LAT2, do not accumulate in skeletal muscle in PET and SPECT, respectively (8,40).
CONCLUSION
In this study, by means of an in vitro transport assay using established mammalian cell lines stably expressing human LAT1 and human LAT2, we have shown that FAMT is a high-affinity substrate of LAT1. Distinct from the other amino acid PET tracers, FAMT is selective to LAT1 and interacts less with LAT2 because of its α-methyl moiety. This property of FAMT contributes to highly tumor-specific accumulation of 18F-FAMT in PET.
DISCLOSURE STATEMENT
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Michiko Minobe for technical assistance. This work was supported in part by a Grant-in-Aid for scientific research on priority areas of “Transportsome” and Grant-in-Aid 22300334 from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Grant-in-Aid for scientific research from the Japan Society for the Promotion of Science; the Hyogo prefecture COE Program; the A-STEP from the Japan Science and Technology Agency; the TR project from the New Energy and Industrial Technology Development Organization (NEDO), Japan; and the Ajinomoto Amino Acid Research Program. No other potential conflict of interest relevant to this article was reported.
Footnotes
↵* Contributed equally to this work.
Published online Jun. 28, 2012.
- © 2012 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication January 11, 2012.
- Accepted for publication April 4, 2012.