Abstract
The large, neutral L-type amino acid transporters (LAT1–LAT4) are sodium-independent transporters that are widely distributed throughout the body. LAT expression levels are increased in many types of cancer, and their expression increases as cancers progress, leading to high expression levels in high-grade tumors and metastases. Because of the key role and overexpression of LAT in many types of cancer, radiolabeled LAT substrates are promising candidates for nuclear imaging of malignancies that are not well revealed by conventional radiotracers. The goal of this study was to examine the structure–activity relationships of a series of 18F-labeled amino acids that were predicted to be substrates of the LAT transport system. Methods: Using a photocatalytic radical fluorination, we prepared a series of 11 fluorinated branched-chain amino acids and evaluated them and their nonfluorinated parents in a cell-based LAT affinity assay. We radiofluorinated selected branched-chain amino acids via the same radical fluorination reaction and evaluated tumor uptake in U-87 glioma xenograft–bearing mice. Results: Structure–activity relationship trends observed in a LAT affinity assay were maintained in further in vitro studies, as well as in vivo using a U-87 xenograft model. LAT1 uptake was tolerant of fluorinated amino acid stereochemistry and chain length. PET imaging and biodistribution studies showed that the tracer (S)-5-18F-fluorohomoleucine had rapid tumor uptake, favorable in vivo kinetics, and good stability. Conclusion: By using an in vitro affinity assay, we could predict LAT-mediated cancer cell uptake in a panel of fluorinated amino acids. These predictions were consistent when applied to different cell lines and murine tumor models, and several new tracers may be suitable for further development as oncologic PET imaging agents.
A hallmark of cancer is a metabolic reprogramming of cells that enables increased nutrient uptake and altered metabolism required for rapid growth and division (1). In addition to the increased uptake of glucose by cancer cells, most malignancies also display an increased uptake of amino acids that are required for accelerated protein synthesis, alternative modes of energy production, or cell signaling (2). Because amino acids have traditionally shown low basal uptake in tissues with high glucose uptake such as brain, inflammation, or infection, 18F-amino acid radiotracers can complement 18F-FDG PET imaging in the visualization and quantification of various malignancies (3). Of interest to cancer biology are the L-type amino acid transporters (LATs) and, more specifically, LAT1, which plays a key role in transporting large, neutral amino acids (4,5). LAT1 is overexpressed in a wide variety of human tumors, and its expression level correlates with tumor cell proliferation, angiogenesis, and poor patient outcomes (6). Thus, LAT1 represents a promising target for cancer imaging via PET. In particular, PET radiotracers targeted toward the LAT system are of value in the imaging and monitoring of gliomas because LAT is highly expressed at the blood–brain barrier (BBB), thus supplying the brain with essential amino acids (7). This expression at the BBB allows LAT-targeted radiotracers to reach the entire brain volume, as well as glioblastomas with intact BBB membranes (8). The key role that LAT transport plays in both cancer and BBB crossing renders the study and discovery of LAT-targeting amino acid radiotracers a valuable medical endeavor.
To date, several amino acid LAT substrates have displayed promise as oncologic PET tracers (Fig. 1). Most of these are α-hydrogen-α-amino acids, have an (S) stereochemical configuration, and contain an aromatic ring. Tracers such as O-(2-18F-flurorethyl-l-tyrosine (18F-FET) (9) and 6-18F-fluoro-3,4-dihydroxy-l-pheynylalanine (18F-FDOPA) (10) have been used for imaging of various cancers in humans and are particularly useful for neurooncologic imaging (11,12). In the case of aromatic LAT substrates, replacement of the α-hydrogen with a methyl group, as in l-3-18F-fluoro-α-methyl tyrosine (18F-FAMT) (13) and 3-123I-iodo-α-methyl-l-tyrosine (123I-IMT) (14), does not appear to affect LAT affinity and, in the case of 18F-FAMT, can impart selectivity for LAT1 over LAT2 (15). Aliphatic radiolabeled LAT substrates include l-11C-methionine (11C-MET) (16), (S)-2-amino-7-18F-fluoroheptanoic acid ((S)-18F-FAHep) (17), and (S)-5-18F-fluoroleucine (18). Of these, only 11C-MET has been used in humans, and although (S)-18F-FAHep shows promise for brain tumor imaging, (S)-5-18F-fluoroleucine shows significant in vivo defluorination, limiting its further development. Despite these and other discoveries, the number of systematic structure–activity relationship (SAR) studies related to LAT-based radiotracers remains limited. A recent study by Nagamori et al. indicates that a β-amino acid (l-β-homoleucine) and d-amino acids (d-leucine, and d-homoleucine) can act as effective LAT substrates, yet these findings have not been translated to in vivo radiotracer studies (19). Bouhlel et al. have also reported in select cases that (R)-configured amino acids display LAT-mediated transport (20). Furthermore, although any amino acid radiotracer can potentially be synthesized by labeling the carboxylate terminus with 11C, yielding an unsubstituted amino acid (21), a formal comparison of unsubstituted versus fluorinated amino acids in cellular amino acid transport has, to the best of our knowledge, not yet been performed. As with drug discovery, SAR can be a powerful tool in the rational design of optimized radiotracers, and in the current study, we apply a SAR approach to the study of LAT substrates and their potential as oncologic radiotracers.
Radiolabeled amino acid tracers targeting LAT.
We recently reported the radiosynthesis of several 18F-labeled branched-chain amino acids (18F-FBCAAs) via a photocatalyzed radical fluorination (22). Here, we use this fluorination platform to synthesize an extended panel of 18F-labeled BCAAs. Evaluation of the LAT-mediated transport of these BCAAs and their potential as tumor imaging agents was performed by both in vitro and in vivo methods. We find that the major transporter responsible for 18F-FBCAA uptake is the LAT system and that LAT affinity correlates with the ability of the 18F-FBCAAs to be taken up in U-87 xenograft tumors.
MATERIALS AND METHODS
Radiosynthesis
18F-F2 was generated from a TR13 cyclotron by a 2-part irradiation process. 18O-O2/Ar was loaded to the target chamber and irradiated at 25 μA/min for 15 min. 18O-O2 was trapped cryogenically and recycled. Then, the target was filled with F2/argon and irradiated again at 20 μA/min for 5 min. The target was emptied to the synthesis module by an argon flow. The 18F-FBCAAs were radiosynthesized in a manner similar to that previously described (22). Briefly, 18F-F2 was bubbled through a solution of sodium dibenzenesulfonamide (40 mg) in 600 μL of CH3CN and 200 μL of H2O. The reaction mixture was loaded on a C18 long SepPak (WAT023635; Waters). After washing with 5 mL of H2O and 0.6 mL of CH3CN, 18F-N-fluorobenzenesulfonimide (NFSI) was eluted with 1.2 mL of CH3CN. The 18F-NFSI solution was added to a slurry of the substrate as a trifluoroacetic acid salt (typically 10–15 mg) and sodium decatungstate (5 mg, 2.0 μmol) in 200–400 μL of H2O and mixed briefly. The solution was then loaded onto a microfluidic reactor (XXL-ST-02; Little Things Factory) on a transilluminator (89131-468, 365 nm; VWR) shown in Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org) and photoirradiated for 45 min. After this time, the solution was removed and the photoreactor was washed with CH3CN (5 mL). The resulting solution was loaded onto a strong cation-exchange cartridge (500 mg of resin, SPE-P0005; SiliCycle), and the cartridge was washed with CH3CN (10 mL) followed by H2O (10 mL). The 18F-FBCAAs were then eluted from the cartridge with 1 mL aliquots of 150 mM NaHCO3, yielding a mixture of fluorinated product and starting material. Analytic high-performance liquid chromatography was performed on a Phenomenex Luna C18 (4.6 × 150 mm, 1 mL/min) using a gradient of 100% solvent A (0.1% trifluoroacetic acid in H2O) to 100% solvent B (0.1% trifluoroacetic acid in CH3CN) over 15 min.
RESULTS
Chemistry
When necessary, resolution was performed by a 2-step N-acylation and enzymatic deacylation using porcine kidney acylase to yield the (S)-isomer of the BCAA according to Chenault et al. (23). The BCAAs were fluorinated via a photocatalytic C-H fluorination procedure (24–26) as depicted below (Fig. 2) and described in the supplemental materials.
(A) Direct fluorination of BCAAs using photochemical radical fluorination. (B) Panel of 22 parent and fluorinated BCAAs used in this study.
LAT Uptake Studies
To quantify the ability of the BCAAs to be transported by the LAT system, a leucine transport assay was used, which measures the antagonism of 3H-leucine uptake in CHO-K1 cells (4). The results from this study (Fig. 3) add to the work of Nagamori et al., who previously performed LAT transport SAR in HeLa S3 cells, albeit at a single concentration (1 mM) (19). We found that LAT transport was optimal for α-amino acids and that a 1-carbon increase in aliphatic chain length was tolerated (Fig. 3, entries 1–3). In line with results reported by Nagamori et al. (19), we found that LAT was relatively tolerant of the absolute stereochemistry of α-amino acids, with similar LAT affinity for both enantiomers (Fig. 3, entries 1, 2, 4, and 5). However, we found a clear difference in LAT affinity between enantiomeric β-amino acids (Fig. 3, entries 6 and 10). Notably, fluorination of the BCAAs at the branched position did not greatly affect LAT affinity for optimal substrates but decreased affinity in poorer substrates (Fig. 3, entries 6, 7, and 8). In accord with findings reported by Bouhlel et al. (17), we found that α-methylation greatly diminished LAT affinity (Fig. 3, entries 8 and 9). These findings confirm that α-methylation in aliphatic amino acids, as opposed to aromatic amino acids, decreases LAT affinity and that the use of the more desirable 18F radiolabel, as opposed to 11C, should not impair LAT transport for higher-affinity LAT substrates.
LAT affinity of BCAA/FBCAA pairs in CHO cells. IC50 is for uptake of 3H-leucine into CHO-K1 cells in Na+-free medium.
The structure of mammalian LAT1 is as yet unknown; however, homology modeling based on the x-ray structure of the arginine/agmatine transporter AdiC from Escherichia coli displays crucial substrate binding via hydrogen bonds at the acid oxygens and amino group (27). To shed light on the effect of fluorination on the BCAAs, we measured the pKa of 3 BCAA/FBCAA couples: (S)-HL/(S)-5-fluorohomoleucine (FHL), pregabalin/5-FPregab, and (S)-αMHL/(S)-5-FαMHL (Fig. 4). In all cases, fluorination lowered the pKa values of the acid and base slightly. Although the effect of fluorination on ammonium pKa was relatively constant among the compounds analyzed (ΔpKa ∼ −0.40), fluorination decreased the pKa of the carboxylic acid function by about 0.1 for pregabalin and (S)-αMHL but had almost no effect (ΔpKa ∼ −0.03) for (S)-HL. The difference between carboxylic acid pKa for pregabalin and 5-FPregab may contribute to the decrease in LAT affinity for 5-FPregab, which was not observed between (S)-HL and (S)-5-FHL.
Measured pKa values for selected BCAA/FBCAA pairs. pKa acid = carboxylic acid; pKa base = protonated amine.
Radiosynthesis
The 2-step radiosynthesis of 18F-FBCAAs was adapted from our previous report (22). First, 18F-NFSI (28) was produced by passing 18F-F2 through the CH3CN-H2O solution of sodium dibenzenesulfonamide. This was followed by loading a mixture of BCAA, sodium decatungstate, and 18F-NFSI in CH3CN-H2O solution onto a microfluidic reactor placed on a transilluminator and irradiated at a λ of 365 nm at ambient temperature for 45 min. Purification was performed via a cation-exchange cartridge. In all cases, the 18F-FBCAAs were synthesized and purified in less than 60 min. The radiochemical parameters of the 18F-FBCAAs are shown in Figure 5. The use of 18F-F2 gas results in radiotracers with low molar activities (∼3–16 MBq/μmol), and although this may not be suitable for all purposes, fluorinated amino acid radiotracers of this molar activity range have previously been effective in clinical studies (29).
Radiochemical parameters for radiosynthesis of 18F-FBCAAs evaluated in vivo in this study. All numbers are n = 3 or greater, with exception of (S)-6-18F-FHHL, which was synthesized with n = 2. aPreviously reported (22). RCY = radiochemical yield of isolated final products from 18F-NFSI → 18F-FBCAA transformation; RCP = radiochemical purity as determined by radiodetected reversed-phase high-performance liquid chromatography; MA = molar activity in MBq/μmol as determined by 1H NMR spectroscopy (more detail is in supplemental materials).
Competition Assay
We further examined the uptake of a selected panel of 5 18F-FBCAAs in a PSMA (prostate specific membrane antigen)-negative prostate cancer cell line (PC3). Uptake of the 18F-FBCAAs was measured with or without the addition of a competitive amino acid substrate to interrogate the specificity of FBCAA uptake. Here, the uptake of all 5 tracers was blocked by l-leucine and 2-amino[2.2.1]heptane-2-carboxylic acid (BCH), which are LAT-specific inhibitors (30). The uptake was not blocked by l-glutamate, l-serine, or N-methyl α-aminoisobutyric acid, indicating that these new tracers are not transported by excitatory amino acid transporters, system xCT, system A, or system ASC (31). Uptake of each tracer was blocked to a similar extent by BCH and l-leucine, indicating a similar role of LAT in their transport. Conversely, transport of (S)- and (R)-5-18F-FHL was not affected by excess l-phenylalanine, which largely inhibited uptake of the other 18F-FBCAAs, indicating a contribution of the other transport systems to the uptake of these radiotracers (Fig. 6). Notably, uptake in PC3 cells was the highest for (S)-5-18F-FHL, followed by (R)-5-18F-FHL, (S)-5-18F-FBAHL, (R)-5-18F-FBAHL, and (S)-4-18F-FαML, in agreement with the data presented in Figure 3.
In vitro uptake of (S)-5-18F-FHL, (S)-5-18F-FBAHL, (S)-4-18F-FαML, (R)-5-18F-FHL, and (R)-5-18F-FBAHL in PC3 prostate cancer cells in presence of competitive inhibitors (10 mM) of amino acid transport. Uptake data are expressed as percentage relative to control condition (no competition), and values are normalized to total numbers of cells per well. MeAIB = N-methyl α-aminoisobutyric acid.
In Vivo Biodistribution
We elected to further study the LAT-based tumor uptake of a panel of 18F-FBCAAs in NSG (NOD Scid gamma) mice xenografted with U-87 tumors based on our in vitro data. As would be expected from amino acid radiotracers, accumulation was pronounced in the pancreas and kidney, with excretion mainly via the urine (Fig. 7, Supplemental Table 1). All compounds displayed good metabolic stability as evidenced by low bone uptake, with the exception of 5-18F-FPregab, which displayed slightly elevated bone accumulation (3.36 ± 0.55 percentage injected dose [%ID]/g). (S)-5-18F-FHL, (S)-6-18F-FHHL, and (R)-5-18F-FHL had higher tumor uptake than (S)-5-18F-FBAHL. 5-18F-FPregab, (S)-4-18F-FαML, and (S)-5-18F-FαMHL had much lower tumor uptake. These results were consistent with our findings in cell uptake assays. (S)-5-18F-FHL, (S)-6-18F-FHHL, and (R)-5-18F-FHL displayed brain uptake comparable to that of (S)-5-18F-FBAHL, 5-18F-FPregab, (S)-4-18F-FαML, and (S)-5-18F-FαMHL, consistent with LAT expression patterns at the BBB (7).
In vivo biodistribution in NSG mice bearing U-87 xenograft tumors with (S)-5-18F-FHL, (S)-6-18F-FHHL, (R)-5-18F-FHL, (S)-5-18F-FBAHL, (S)-5-18F-FPregab, (S)-4-18F-FαML, and (S)-5-18F-FαMHL, and ratios of selected tracer uptake in target tissues (inset). All data were obtained 60 min after injection. Data are %ID/g (mean ± SD, n ≥ 3).
DISCUSSION
The LAT family is responsible for the transport of large, neutral amino acids such as phenylalanine, leucine, and isoleucine (4). Among the LAT family, LAT1 is the most extensively overexpressed in many types of tumors and their metastatic lesions, with expression of LAT1 correlating with tumor cell proliferation, angiogenesis, and poor prognosis (32). The overexpression of LAT1 and other members of the LAT transporter family in cancers and the resulting accumulation of neutral, hydrophobic amino acids makes the use of 18F-labeled leucine-based radiotracers an attractive approach to PET imaging in oncology.
In this report, we applied a photocatalytic fluorination reaction (24,25,33) to rapidly produce and evaluate a series of fluorinated BCAAs as PET radiotracers. In vitro LAT affinity measured in CHO-K1 cells (Fig. 3) demonstrated a fair degree of substrate promiscuity because both (S)- and (R)-α-amino acids were well transported into cells. Indeed, both stereoisomers of leucine and HL displayed similar LAT affinity in our system (half-maximal inhibitory concentration [IC50] ∼ 15 μM). β-amino acids were less ideal substrates, as (S)-BAHL displayed approximately an order of magnitude poorer affinity (IC50 ∼ 150 μM) toward LAT than either (S)- or (R)-HL, and (R)-BAHL displayed little LAT affinity (IC50 > 1,000 μM). Longer chains were tolerated, as (S)-HHL was transported to approximately the same extent as the leucine and HL series. We found that α-methylation resulted in a significant decrease in LAT affinity, with αML (IC50 ∼ 400 μM) and αMHL (IC50 > 1000 μM) in line with previous studies (17). Fluorination at the branched position in these amino acids resulted in negligible changes in LAT affinity for well-accepted substrates (L, HL, HHL) but was less tolerated in moderate substrates (BAHL, αML, αMHL). The central nervous system amino acid drug pregabalin (34), which is carried past the BBB via LAT transport, displayed an IC50 of about 190 μM, in good agreement with a previous report (35). However, fluorination of this substrate to afford 5-FPregab resulted in complete loss of LAT affinity.
Radiofluorination provided 18F-FBCAAs in good radiochemical yield and purity, and in vitro uptake of these radiolabeled amino acids was examined in a PSMA-negative prostate cancer cell line (PC3). In this assay, we found that radiotracer uptake correlated with findings from the LAT affinity assay. We further showed that the uptake was efficiently blocked by BCH, l-valine, l-leucine, and, to a lesser extent, l-Met but not by N-methyl α-aminoisobutyric acid, l-Ser, or l-Glu, indicating that the primary in vitro uptake mechanism of these 18F-FBCAAs was via the L-type system. In the case of poorer LAT substrates such as (S)-5-18F-FBAHL, (R)-5-18F-FBAHL, and (S)-4-18F-FaML, in addition to antagonism by leucine, valine, and BCH, uptake is strongly antagonized by l-Phe, indicating that although LAT plays a role in cellular uptake, other transport mechanisms may be in operation for these tracers. We then selected a series of 18F-FBCAAs with varying LAT affinity and examined their biodistribution in mice bearing U-87 cell line xenografts predicted to overexpress LAT (36). We found that accumulation correlated well with LAT affinity trends, with (S)-5-18F-FHL, (R)-5-18F-FHL, and (S)-6-18F-FHHL showing the highest tumor uptake (Fig. 7, Supplemental Table 1) at 60 min after injection. As predicted, (S)-5-18F-FBAHL showed lesser tumor accumulation and 5-18F-FPregab, (S)-4-18F-FαML, and (S)-5-18F-FαMHL showed a further decrease in tumor accumulation.
Finally, the imaging potential of several 18F-FBCAAs was examined in a PET study. Dynamic PET images of U-87 tumors in NSG mice at 10 min with (S)-5-18F-FHL, 5-18F-FPregab, (S)-4-18F-FαML, and (S)-5-18F-FαMHL were obtained and confirmed the results shown in Figure 7. (S)-5-18F-FHL showed good tumor visualization at 10 min (indicated by orange arrow), whereas the LAT-inactive substrate (S)-5-18F-FαMHL (LAT affinity IC50 > 1,000 μM) showed no tumor uptake at this time point (Fig. 8). Interestingly, there were notable differences between (S)-4-18F-FαML and 5-18F-FPregab (both LAT affinity IC50 > 1,000 μM), as the tumor visualized with (S)-4-18F-FαML showed much better contrast than that visualized with 5-18F-FPregab. This finding may be attributable to differences in LAT affinity not revealed by the uptake assay, tumor uptake of (S)-4-18F-FαML by transporters other than LAT, or differences in (S)-4-18F-FαML and 5-18F-FPregab clearance and metabolism.
PET images at 10 min for U-87 xenograft–bearing mice with (S)-5-18F-FHL, (S)-5-18F-FαMHL, (S)-4-18F-FαML, and 5-18F-FPregab. The orange arrow indicates the site of tumor.
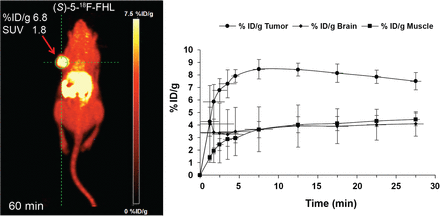
A closer examination of the in vivo behavior of (S)-5-18F-FHL showed rapid tumor uptake, plateauing at around 10 min and then remaining stable until 30 min of acquisition time, as evidenced by time–activity curves (Fig. 9). The average values of last time frames (uptake values at plateau, i.e., %ID/g, ∼7.4) corresponded well to the total averages for dynamic scans (at 30 min).
(A) In vivo dynamic PET imaging of (S)-5-18F-FHL in NSG mouse bearing U-87 xenograft tumor at 60 min after injection. (B) Time–activity curve of (S)-5-18F-FHL in tumor, brain, and muscle over 30 min after injection, n = 3.
The tumor and brain uptake of (S)-5-18F-FHL were similar to that of the clinical LAT radiotracer 18F-FET. In a previous report, 18F-FET showed an uptake of 6.37 ± 1.67 %ID/g (colon carcinoma tumor) and 2.17 ± 0.64 %ID/g (brain) at 60 min after injection in tumor-bearing nude mice (9). These values are similar to those obtained with (S)-5-18F-FHL, which showed uptake of 5.29 ± 0.84 %ID/g (U-87 tumor) and 1.63 ± 0.3 %ID/g (brain) at 60 min after injection.
CONCLUSION
Using a photocatalytic fluorination platform, we were able to rapidly access several fluorinated and radiofluorinated BCAAs. An uptake study in CHO-K1 cells revealed SAR of BCAA uptake by the LAT system and allowed us to select compounds for radiofluorination. A panel of selected 18F-FBCAAs was synthesized, and an in vitro competition assay further demonstrated that they are LAT-specific substrates. Further in vivo studies confirmed that LAT-mediated uptake in tumor, brain, and other organs correlated with the SAR uncovered in the uptake study. From this study, we were able to identify several promising 18F-FBCAA tracers that will be subjected to further study in models of glioma, prostate cancer, and multiple myeloma.
DISCLOSURE
This work was supported by an NSERC Discovery Grant, an MSFHR Innovation to Commercialization Grant, and a MSFHR Career Investigator Award to Robert Britton, a Hoffmann-La Roche Fellowship (RPF) to Matthew Nodwell, a CIHR Operating Grant and a NSERC CREATE IsoSiM Stipend to Milena Čolović, the BC Leading Edge Endowment Fund to François Bénard, and a Canadian Cancer Society Research Institute Innovation Grant to Paul Schaffer. TRIUMF receives federal funding via a contribution agreement with the National Research Council of Canada. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank the TRIUMF TR13 cyclotron team, in particular David Prevost, Linda Graham, and Samuel Varah.
Footnotes
Published online Jan. 25, 2019.
- © 2019 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication September 17, 2018.
- Accepted for publication November 30, 2018.