Abstract
Affibody molecules are a recently developed class of targeting proteins based on a nonimmunoglobulin scaffold. The small size (7 kDa) and subnanomolar affinity of Affibody molecules enables high-contrast imaging of tumor-associated molecular targets, particularly human epidermal growth factor receptor type 2 (HER2). 99mTc as a label offers advantages in clinical practice, and earlier studies demonstrated that 99mTc-labeled recombinant Affibody molecules with a C-terminal cysteine could be used for HER2 imaging. However, the renal retention of radioactivity exceeded tumor uptake, which might complicate imaging of metastases in the lumbar region. The aim of this study was to develop an agent with low renal uptake and preserved tumor targeting. Methods: A series of recombinant derivatives of the HER2-binding ZHER2:342 Affibody molecule with a C-terminal chelating sequence, –GXXC (X denoting glycine, serine, lysine, or glutamate), was designed. The constructs were labeled with 99mTc and evaluated in vitro and in vivo. Results: All variants were stably labeled with 99mTc, with preserved capacity to bind specifically to HER2-expressing cells in vitro and in vivo. The composition of the chelating sequence had a clear influence on the cellular processing and biodistribution properties of the Affibody molecules. The best variant, 99mTc-ZHER2:V2, with the C-terminal chelating sequence –GGGC, provided the lowest radioactivity retention in all normal organs and tissues including the kidneys. 99mTc-ZHER2:V2 displayed high uptake of radioactivity in HER2-expressing xenografts, 22.6 ± 4.0 and 7.7 ± 1.5 percentage injected activity per gram of tissue at 4 h after injection in SKOV-3 (high HER2 expression) and DU-145 (low HER2 expression) tumors, respectively. In both models, the tumor uptake exceeded the renal uptake. Conclusion: These results demonstrate that the biodistribution properties of recombinant 99mTc-labeled Affibody molecules can be optimized by modification of the C-terminal cysteine-containing chelating sequence. 99mTc-ZHER2:V2 is a promising candidate for further development as a diagnostic radiopharmaceutical for imaging of HER2-expressing tumors. These results may be useful for the development of imaging agents based on other Affibody molecules and, hopefully, other scaffolds.
Overexpression of human epidermal growth factor receptor type 2 (HER2) provides tumors with a growth advantage and is a part of the malignant phenotype (1). The use of HER2-targeting monoclonal antibodies or tyrosine kinase inhibitors increases survival of patients with disseminated HER2-expressing breast cancer (2,3). Detection of HER2 expression in vivo is necessary to stratify patients for HER2-targeting therapy (4–6). Molecular imaging has the potential to visualize HER2 expression in both primary tumors and metastases in a single noninvasive procedure (7). Several types of HER2-targeting imaging agents have been evaluated for this purpose (7,8). Affibody molecules (Affibody AB), small (7 kDa) engineered affinity proteins, constitute a promising class of tracers for imaging (9,10). Several variants of the ZHER2:342 anti-HER2 Affibody molecule have been labeled with various nuclides and enabled high-contrast imaging of HER2-expressing xenografts in mice (9,10). Moreover, 111In- and 68Ga-labeled variants of this molecule have been used for clinical imaging of HER2-expressing metastases (11).
99mTc (half-life, 6 h; γ-energy, 140.5 keV) is a commonly used nuclide in molecular imaging applications because of its photon energy (nearly ideal for SPECT), low cost, excellent availability, and low absorbed-dose burden to the patient. Recently, several approaches to label the HER2-binding Affibody molecule ZHER2:342 and its derivatives with 99mTc have been evaluated (12–21). Variants and positioning of histidine tags, as well as mercaptoacetyl- or cysteine-containing peptide-based chelators, were evaluated for the coordination of 99mTc. Results from the biodistribution studies demonstrated that the derivatives of ZHER2:342 were cleared from the body mainly via glomerular filtration, followed by a high degree of renal reabsorption leading to a high level of renal retention of radioactivity when residualizing labels were used. Moreover, the use of hexahistidine tags for labeling and purification was shown to cause an unwanted, elevated hepatic uptake of radioactivity (12,19,22,23). The evaluation of peptide-based chelators positioned at the N-terminus of the tracer revealed that the amino acid composition of the N-terminus was critical for the biodistribution profile in terms of liver uptake and the extent of hepatobiliary excretion. The use of amino acids with hydrophilic (polar or charged) side chains in the N-terminal chelators provided reduced liver uptake and low levels of hepatobiliary excretion (14,16–18,22), whereas the composition of the C-terminus was shown to be less influential in that aspect (23). However, in agreement with previously published data on chelator stability and biodistribution of 99mTc-labeled proteins (24–26), the use of an N3S cysteine–based chelator at the C-terminus provided appreciably less release of 99mTc-pertechnetate in the circulation than did the SN3 chelator at the N-terminus (15,19,21). Furthermore, Ala1,Glu2 at the N-terminus has been shown to be associated with low hepatic uptake and low hepatobiliary excretion (19,21–23). A more detailed summary of previous results and the influence of chelators on the biodistribution of Affibody molecules is provided in the supplemental materials (available online only at http://jnm.snmjournals.org).
On the basis of our observations, we hypothesized that Affibody molecules containing the amino acid sequences AEN− at the N-terminus and the –GGGC, –GGSC, –GGEC, or –GGKC peptide–based chelators (Fig. 1) at the C-terminus could be labeled with 99mTc, with preserved binding specificity to HER2; would be stable in the circulation; would be cleared from blood circulation predominantly by renal excretion; and would yield low renal retention of radioactivity.
General structure of N3S chelator formed by C-terminal cysteine and adjacent amino acids.
To verify this hypothesis, 4 ZHER2:342 Affibody variants were generated (Fig. 2), labeled with 99mTc, and evaluated in vitro and in vivo. For comparison, an anti-HER2 Affibody molecule, ZHER2:V1, which is homologous to the previously studied PEP05352 (21) (an Affibody molecule displaying low retention of 99mTc radioactivity in the kidneys) but that does not contain any scaffold modifications or C-terminal glycine, was included in the study.
Alignment of studied HER2-binding Affibody molecules. Positions of α-helices 1 through 3 are indicated by boxes. Peptide-based chelators are marked by bold font, and variable amino acids are marked red.
MATERIALS AND METHODS
Materials
α-d-gluconic acid sodium salt and ethylenediaminetetraacetic acid were from Sigma-Aldrich, and tin(II)-chloride dehydrate and pyridine were from Fluka Chemika. Phosphate-buffered saline (PBS), pH 7.4, was produced in-house. 99mTc was obtained as pertechnetate from an Ultra-TechneKow generator (Covidien) eluted with sterile 0.9% sodium chloride (Covidien). An automated γ-counter (Perkin-Elmer) was used to measure the radioactivity. Yield, radiocolloid content, and radiochemical purity of the labeled Affibody constructs were analyzed using 150-771 Dark Green, Tec-Control Chromatography strips from Biodex Medical Systems, as previously described (27). NuPAGE 10% Bis-Tis gels (Invitrogen) were used for analysis of conjugates and in vitro stability studies. The Cyclone Storage Phosphor System and the OptiQuant image analysis software (Perkin-Elmer) were used to measure the radioactivity distribution on chromatography strips and polyacrylamide gel electrophoresis (PAGE) gels.
Preparation of Affibody Molecules
A detailed description of the production, purification, and characterization of the ZHER2:V1–ZHER2:V5 Affibody molecules is provided elsewhere (28), and some relevant properties are presented in Table 1. All variants are characterized by high melting points (over 63°C) and high affinity (140–260 pM) to HER2. Purified proteins were divided in aliquots of 100 μg and freeze-dried for storage.
Properties of Affibody Molecules
Radiolabeling and In Vitro Stability
Radiolabeling was performed using the optimized 2-vial kit method described by Ahlgren et al. (27). For biodistribution studies, conjugates were purified using disposable NAP-5 columns (GE Healthcare) preequilibrated with PBS. After purification, the purity was evaluated using instant thin-layer chromatography and cross-validated using radio–sodium dodecyl sulfate (SDS)-PAGE.
For serum stability studies, freshly labeled ZHER2:V1-ZHER2:V5 (10 μL) was diluted in a serum sample (240 μL) to a concentration similar to the concentration in blood at the moment of injection, incubated for 1 h at 37°C, and analyzed using radio–SDS-PAGE in 2-(N-morpholino)ethanesulfonic acid buffer (200 V constant). A sample of pertechnetate was run in parallel as reference.
In Vitro Cell Studies
The HER2-expressing ovarian cancer cell line SKOV-3, 1.2 × 106 HER2 receptors per cell (29), and the prostate cancer cell line DU-145, 5 × 104 HER2 receptors per cell (Jennie Malmberg, unpublished data, 2010), were used in cell studies. The specificity of radiolabeled Affibody molecules binding to these cells and cellular retention and internalization of radioactivity were evaluated, as described by Wållberg and Orlova (30).
In Vivo Studies
Biodistribution Studies.
The animal study was approved by the Local Ethics Committee for Animal Research. In comparative biodistribution studies, non–tumor-bearing NMRI mice were used. The mice were randomly divided into groups of 4. Three groups were intravenously injected with each conjugate (1 μg of Affibody ligand, ∼480 kBq in 100 μL of PBS) and euthanized by an intraperitoneal injection of ketamine (Ketalar; Pfizer) and xylazine (Rompun; Bayer) at 1, 4, and 24 h after injection. Blood and organ samples were collected and weighed, and their radioactivity content was measured. The organ uptake values were calculated as percentage injected activity per gram of tissue (%IA/g).
The tumor-targeting properties of the conjugates with the most favorable biodistribution, 99mTc-ZHER2:V1 and 99mTc-ZHER2:V2, were studied in mice bearing SKOV-3 and DU-145 xenografts. For inoculation, 5 × 106 DU-145 cells in Matrigel (BD Biosciences) or 10 × 106 SKOV-3 cells were implanted on the right hind leg of immunodeficient mice. Animals (4 mice per data point) were injected with 80 kBq of 99mTc-ZHER2:V1 or 99mTc-ZHER2:V2 in 100 μL of PBS. The injected protein dose was 1 μg for mice bearing SKOV-3 tumors and 0.3 μg for DU-145 tumors. Biodistribution and tumor targeting were studied at 4 h after injection. To show specificity of 99mTc-ZHER2:V2 targeting in vivo, 1 group of mice with SKOV-3 and 1 with DU-145 xenografts were preinjected with 1 mg of nonlabeled ZHER2:342 at 40 min before the injection of 99mTc-ZHER2:V2. The animals were sacrificed at 4 h after injection, and the radioactivity concentration in blood and tumors was measured.
γ-Camera Imaging.
In vivo imaging was performed to obtain a visual confirmation of the biodistribution data. SKOV-3 and DU-145 xenograft–bearing mice were injected with 99mTc-ZHER2:V1 or 99mTc-ZHER2:V2. The mice bearing SKOV-3 xenografts were injected with 0.9 MBq (2 μg), and mice bearing DU-145 xenografts with 0.85 MBq (0.3 μg). Immediately before imaging, at 4 h after injection, the animals were sacrificed, and the urinary bladders were excised. The imaging experiment was performed using a Infinia γ-camera (GE Healthcare) equipped with a low-energy high-resolution collimator. Static images (30 min) were obtained with a zoom factor of 2 in a 256 × 256 matrix.
RESULTS
Radiolabeling and In Vitro Stability
The results of radiolabeling and stability tests are presented in Table 2. The use of an optimized procedure (27) provided labeling yields over 90% for all conjugates and a radiocolloid content below 3%. Purification using disposable NAP-5 columns provided radiochemical purity over 98%.
Labeling of Affibody Molecules
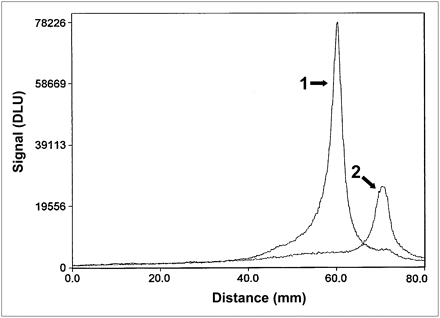
Figure 3 shows a representative radioactivity distribution in SDS-PAGE gel after incubation in murine blood plasma at 37°C for 60 min. The main peak corresponded to the monomeric Affibody molecule. No peaks indicating aggregation of Affibody molecules or transchelation of the radionuclide to blood plasma proteins were observed. The only other observed radioactivity peak corresponded to low-molecular-weight compounds, such as 99mTc-pertechnetate. This peak did not contain more than 3.5% of the total activity at the end of the stability test (Table 2).
Representative SDS-PAGE analysis of stability of 99mTc-labeled Affibody molecules (99mTc-ZHER2:V2) in serum. 1. 99mTc-ZHER2:V2 incubated in murine serum at 37°C for 1 h; 2. 99mTcO4− used as marker for low-molecular-weight compounds. Signal, measured as digital light units, is in proportion to radioactivity in given point of lane in SDS-PAGE gel. DLU = digital light units.
In Vitro Cell Studies
Binding specificity tests (Table 3) indicated that the binding of all 99mTc-labeled Affibody constructs to HER2-expressing cells was receptor-mediated because saturation of the receptors by preincubation with nonlabeled ZHER2:342 significantly decreased the binding of the radiolabeled Affibody molecule.
Specificity of Binding of 99mTc-Labeled Affibody Molecules to HER2-Expressing Cells In Vitro
Cellular Retention and Internalization Experiments.
The results of the cellular retention and internalization experiments are presented in Figure 4. A general feature of all conjugates was the low contribution from internalized radioactivity to the overall cell-bound radioactivity. Instead, the main source of the cell-associated radioactivity was from membrane-bound conjugates. Even after 24 h of incubation, the fraction of internalized radioactivity was in the range of 12%–24% for all conjugates. However, an appreciable difference in cellular retention of the conjugates could be seen. The highest retention was achieved with 99mTc-ZHER2:V5, with 75.0% ± 0.5% of the radioactivity still associated with cells after 24 h, followed by 99mTc-ZHER2:V4 (64.6% ± 0.7% at 24 h). 99mTc-ZHER2:V1 and 99mTc-ZHER2:V3 demonstrated an intermediate retention, 53.3% ± 1.0% and 55.8% ± 2.3%, respectively, after 24 h. The retention level of 99mTc-ZHER2:V2 (47.5% ± 0.4% at 24 h) was the lowest. Still, the difference in cellular retention after 4 h (a time point relevant for in vivo imaging), was not substantial, and 80%–88% of the initially bound radioactivity was still cell-associated at that time point for all conjugates.
Cell-associated radioactivity as function of time after interrupted incubation of SKOV-3 cells with 99mTc-labeled Affibody molecules. Cell-associated radioactivity at time zero after interrupted incubation was considered 100%. Data are presented as average value from 3 dishes ± SD. Error bars might not be seen because they are smaller than point symbols.
In Vivo Studies
Biodistribution Studies.
Data on the comparative biodistribution of the 99mTc-labeled Affibody molecules in male NMRI mice at 1, 4, and 24 h after intravenous injection are presented in Supplemental Table 2. The general pattern of all 5 conjugates was typical for Affibody molecules, with a rapid clearance from the blood, nonspecific compartments, and carcass. The average radioactivity in the gastrointestinal tract, including its content, was below 4% for all conjugates, showing minimal excretion of the conjugates or their radiocatabolites via bile. The low levels of radioactivity uptake in the stomach and salivary gland suggested that the release of 99mTc-pertechnetate in vivo was negligible. There were, however, substantial differences in the biodistribution profiles of Affibody molecules labeled using different N3S chelators. The pulmonary uptake at 4 h after injection was significantly higher for 99mTc-ZHER2:V4 and 99mTc-ZHER2:V5, containing charged amino acids (glutamic acid and lysine, respectively) in the chelating moiety. The hepatic uptake of 99mTc-ZHER2:V5 was 2- to 3-fold higher than the uptake of any of the other conjugates included in the study. Moreover, this conjugate also showed a substantially elevated uptake in the spleen at both 4 and 24 h after injection.
The most remarkable difference between the conjugates was the large difference in renal retention of radioactivity. Already at 1 h after injection, 99mTc-ZHER2:V4 and 99mTc-ZHER2:V5 demonstrated the highest radioactivity accumulation in the kidneys. 99mTc-ZHER2:V1 and 99mTc-ZHER2:V3 showed intermediate values, and 99mTc-ZHER2:V2 demonstrated remarkably low kidney-associated radioactivity, 24 ± 6 %IA/g already this shortly after injection. The renal accumulation of radioactivity was the lowest with 99mTc-ZHER2:V2 at all studied time points. The pattern of renal retention of radioactivity after administration of 99mTc-ZHER2:V4 differed from the pattern seen with the other constructs. At 1 h after injection, the radioactivity in the kidneys was as high as the level seen with 99mTc-ZHER2:V5. However, at 4 h after injection the level seen with 99mTc-ZHER2:V4 was only slightly higher than the levels seen with 99mTc-ZHER2:V1 and 99mTc-ZHER2:V2.
The data concerning the biodistribution of 99mTc-ZHER2:V1 and 99mTc-ZHER2:V2 in mice bearing SKOV-3 and DU-145 xenografts are presented in Tables 4 and 5 and Figure 5. Both conjugates displayed equally good targeting properties in both xenograft models. The presaturation of HER2 receptors with nonlabeled ZHER2:342 caused a statistically significant reduction in radioactivity accumulation in both xenograft models, suggesting specificity of the tumor accumulation of 99mTc-ZHER2:V2 (Fig. 5). In agreement with the data obtained from normal mice, the renal uptake was appreciably reduced in the case of 99mTc-ZHER2:V2 in comparison with 99mTc-ZHER2:V1. The reduction was 3-fold for NMRI nu/nu mice and 3.4-fold for BALB/C mice. In both models, the administration of 99mTc-ZHER2:V2 resulted in a higher uptake of radioactivity in the tumor than in the kidneys, whereas the situation was reversed for 99mTc-ZHER2:V1. Moreover, with 99mTc-ZHER2:V2, significantly less radioactivity accumulated in several other organs, resulting in an overall increase in tumor-to-organ ratios (Tables 4 and 5).
Biodistribution of 99mTc-ZHER2:V1 and 99mTc-ZHER2:V2 Affibody Molecules in Female BALB/c nu/nu Mice Bearing SKOV-3 Xenografts at 4 Hours After Intravenous Injection
Biodistribution of 99mTc-ZHER2:V1 and 99mTc-ZHER2:V2 Affibody Molecules in Male NMRI nu/nu Mice Bearing DU-145 Xenografts at 4 Hours After Intravenous Injection
In vivo binding specificity of 99mTc-ZHER2:V2 in mice bearing ovarian cancer SKOV-3 and prostate cancer DU-145 xenografts at 4 h after injection. Blocked group was subcutaneously preinjected with excess amount of nonlabeled ZHER2:342. Results are presented as percentage of injected activity per gram of tissue (%IA/g).
γ-Camera Imaging.
γ-camera imaging, performed at 4 h after injection (Fig. 6), confirmed the results of the biodistribution experiments. Both conjugates were capable of high-contrast imaging of HER2-expressing tumors. However, the use of 99mTc-ZHER2:V2 provided better contrast than the use of 99mTc-ZHER2:V1.
Imaging of HER2 expression in SKOV-3 ovarian cancer xenografts (high HER2 expression) in BALB/c nu/nu mice and in DU-145 prostate cancer xenografts (moderate HER2 expression) in NMRI nu/nu mice using 99mTc-ZHER2:V1 and 99mTc-ZHER2:V2. Planar γ-camera images were acquired at 4 h after injection. Arrows point to tumors (T) and kidneys (K).
DISCUSSION
The sensitivity of radionuclide molecular imaging depends on the contrast, which in turn depends on the ratio of radioactivity concentration in tumors and healthy tissues. Thus, to obtain maximal contrast the tumor uptake should be increased or the uptake in normal tissues should be decreased as much as possible. In this study, contrast maximization was achieved by minimization of the accumulation in normal tissues. A major focus was on the excretory organs—that is, the liver and kidneys.
The uptake of a protein-based tracer in the liver can occur by several competing and overlapping mechanisms determined by the interaction with different classes of transporter molecules on the hepatocytes. Depending on the molecular characteristics of the radiocatabolites, a radionuclide may be trapped inside the cells (hepatic uptake) or transported into bile (hepatobiliary excretion). A high level of hepatic uptake reduces the sensitivity of imaging and may hamper the detection of liver metastases, whereas hepatobiliary excretion creates diffuse background in the abdomen and decreases the sensitivity of detection of extrahepatic abdominal metastases. Because hexahistidine tags cause an elevated hepatic uptake of Affibody molecules (12,19,22,23) and their use, thus, should be avoided, a novel antiidiotypic purification strategy has been developed and applied to the constructs used in this study (28). Further, N-terminal–placed peptide-based chelators with nonpolar or noncharged side chains were associated with elevated hepatobiliary excretion (13–16). This problem seems to have been solved by the placement of peptide-based chelators at the C-terminus and by improving the design of the N-terminus (AEN-) of the ZHER2:VX constructs, adopted from earlier studies (19,22,31).
An elevated renal uptake is less critical than hepatobiliary excretion but might complicate the detection of metastases in the lumbar area. The use of nonresidualizing radiohalogen labels for labeling of Affibody molecules provides low renal retention due to efficient washout of the lipophilic radiocatabolites (32–35), whereas the use of residualizing radiometal labels leads to high renal retention of radionuclides (22,31,36). Because the internalization of ZHER2:342 and its derivatives by cancer cells is slow (19,22,30,31), residualizing labels are not critical for high tumor uptake shortly after injection, but the tumor uptake is rather determined by the high affinity to HER2. In the kidneys, reabsorption is associated with rapid internalization (37), and the use of nonresidualizing labels would provide the advantage of low renal retention of radioactivity. An optimal chelator should provide stable attachment of 99mTc in the circulation but rapid excretion of radiocatabolites from the kidneys. The design of the ZHER2:VX constructs was based on our previous observation that N3S chelators for the coordination of 99mTc may provide both residualizing and nonresidualizing properties depending on the amino acids adjacent to the thiol-bearing moiety.
The use of amino acids with electron-donating side chains (serine, lysine, glutamate) close to the mercaptoacetyl in the peptide-based chelators at the N-terminus was associated with stabilization of the 99mTc chelate in Affibody molecules (14,16,18), yielding lower release of 99mTc-pertechnetate in the circulation. This stabilization effect has also been reported for homologous thiol-containing chelators by other researchers (38). However, the use of chelators containing several stabilizing side chains caused an elevated renal retention of 99mTc (14,16–18). At the same time, we have found that the renal retention of 99mTc depends on the relative position of the side chains in the chelators (17). It was possible to identify chelators, for example, maESE- (17) and maSKS- (18), which provided a minimal release of free 99mTc-pertechnetate in the circulation but caused low radioactivity retention in kidneys due to the rapid excretion of renal low-molecular-weight radiocatabolites (17). Unfortunately, the exact mechanism for this phenomenon is unclear, and we pursued the minimization of renal uptake by designing a series of Affibody molecules with homologous chelators for the study of this structure–property relation. Cysteine-containing chelators placed at the C-terminus are intrinsically more stable than cysteine placed at the N-terminus because of a more favorable chelating geometry (26). We reasoned that by placing the chelator at the C-terminus, a minimal or no stabilizing effect from electron-donating side chains in the chelating sequence would be required for stability of the 99mTc-complex in the circulation. Furthermore, the use of polar or charged side chains would not be required to suppress the hepatic uptake when placing the chelators at the C-terminus. At the same time, using a minimal number of stabilizing side chains, the renal retention would be reduced, making the 99mTc label nonresidualizing.
We have designed 5 novel Affibody constructs with a –GGXC (X denoting glycine, serine, glutamate, or lysine) and –GSEC (homologous to C-terminus of the previous best PEP05352) C-terminal sequence, which could be used for the chelation of 99mTc. In this way, an amino acid–based chelator was engineered into the Affibody molecules, and no additional processing was required for chelator coupling. The modifications at the C-terminal end insignificantly influenced the affinity of the Affibody molecules to HER2. The radiolabeled Affibody molecules were stable both in vitro (in murine serum) and in vivo. The rapid blood clearance suggests that there was no transchelation to blood proteins from any of the conjugates. Low levels of radioactivity in the stomach and salivary gland indicated that no 99mTc-pertechnetate was released. The in vitro retention and processing experiments demonstrated an apparent influence of the N3S chelators on the cellular retention of the 99mTc-ZHER2:V4 and 99mTc-ZHER2:V5 conjugates, with the –GGEC and –GGKC chelators providing the strongest cellular retention. The cellular retention of 99mTc-ZHER2:V2 was the lowest, suggesting that the −GGGC chelator provides low residualizing properties to 99mTc. However, the slow internalization by HER2-expressing cells, which is typical for ZHER2:342 derivatives (19,22,30,31), leveled out the effect of the chelators. After 4 h of processing (corresponding to an optimal time for imaging), the difference in cellular retention of radioactivity between the 5 conjugates was negligible.
In vivo experiments demonstrated a clear influence from the chelators on the biodistribution of the 99mTc-labeled Affibody molecules. The positioning of a lysine next to cysteine in ZHER2:V5 caused an elevated liver uptake, elevated renal retention, and a slower whole-body clearance. In 99mTc-maKKK-ZHER2:342, the presence of a lysine next to mercaptoacetyl had a similar effect (18). Although the molecular mechanism of this effect is unclear, lysine should probably be avoided in this position. The other conjugates displayed a similar distribution of radioactivity in most of the organs except for the kidneys. The effect of the chelator on kidney retention was striking; at 4 h after injection, the difference in renal accumulations was 19-fold (Supplemental Table 2). At all time points, the lowest level of radioactivity in the kidneys was observed with 99mTc-ZHER2:V2.
99mTc-ZHER2:V2 provided one of the lowest levels of renal radioactivity among all previously evaluated 99mTc-labeled Affibody molecules (12–21). It is likely that the low retention of 99mTc-ZHER2:V2–associated radioactivity in kidneys was due to the absence of stabilizing effects from adjacent electron-donating side chains. The renal uptake of 99mTc-ZHER2:V2 was as low as what was obtained with 99mTc-maGGG-ZHER2:342 (13), but 99mTc-ZHER2:V2 provided a much lower level of hepatobiliary excretion. The advantage of the −GGGC chelator is clearly seen in comparison with 99mTc-CGGG-ZHER2:342 (15). Evidently, 99mTc-ZHER2:V2 is more stable in the circulation than its mirror counterpart—this higher stability manifested as lower levels of radioactivity in the stomach and salivary gland. In addition, 99mTc-ZHER2:V2 displayed lower levels of hepatobiliary excretion due to the optimal design of the N-terminus. Furthermore, excellent biodistribution and targeting properties of 99mTc-ZHER2:V2 was demonstrated in mice bearing HER2-expressing xenografts.
CONCLUSION
The use of a –GGGC N3S chelator at the C-terminal end of anti-HER2 ZHER2:342 Affibody molecules enables stable labeling with technetium, with preserved binding specificity. The substitution of a single amino acid in the chelating sequence can modify the targeting properties of radiolabeled conjugates and the cellular processing and retention of radioactivity by cancer cells. In vivo, modification of the amino acid composition of the chelating moiety can cause a substantial difference in the accumulation of radioactivity in excretory organs, emphasizing the importance of careful optimization of the labeling chemistry in the development of targeting proteins. This information might, hopefully, be useful for other researchers working with scaffold-based targeting proteins.
Acknowledgments
This research was financially supported by grants from the Swedish Cancer Society (Cancerfonden) and the Swedish Research Council (Vetenskapsrådet).
- © 2011 by Society of Nuclear Medicine
REFERENCES
- Received for publication September 24, 2010.
- Accepted for publication December 1, 2010.