Abstract
Theoretically, the degree of 18F-FDG uptake in the glandular tissues of the normal breast can affect the detection of breast cancer. The aim of this prospective study was to investigate relationships among age, menopausal state, and breast density and determine whether they affect 18F-FDG uptake in normal glandular breast tissue. Methods: Among 250 newly diagnosed breast cancer patients, 149 patients (mean age ± SD, 50.9 ± 9.70 y; range, 32–77 y) were analyzed because they had normal contralateral breasts confirmed by MRI, mammography, and 18F-FDG PET examinations. PET images were acquired 60 ± 2 min after the administration of 18F-FDG (5.2 MBq/kg of body weight). The maximum and average standardized uptake value (SUVmax and SUVavg, respectively) of 18F-FDG were calculated in the normal breast. Patients were divided into groups according to qualitative breast density and menopausal state. Descriptive statistics and 2-factorial analysis of covariance were used to assess the effects of qualitative breast density, menopausal state, and age on SUVmax and SUVavg. Pearson χ2 was used to test the relationship between menopausal state and qualitative breast density. Results: The average age of patients with nondense breasts was significantly higher than that of patients with dense breasts (P < 0.01). Also, breast density related to menopausal state (P < 0.05). Dense breasts had an average SUVmax of 1.243 and mean SUVavg of 0.694, whereas nondense breasts had a mean SUVmax of 0.997 and mean SUVavg of 0.592. Analysis of covariance indicated that density and the linear effect of age were significant with regard to both SUVmax and SUVavg. After removing the linear effect of age, menopausal state had no effect on SUVmax and SUVavg. Conclusion: 18F-FDG uptake significantly decreases as age increases and breast density decreases. Age and qualitative breast density are independent factors and significantly affect 18F-FDG uptake for both SUVmax and SUVavg. Menopausal state had no effect on SUVmax and SUVavg.
Breast cancer is the most common cancer among women. Its incidence has also increased in recent decades, with reduction in mortality due to early detection, screening mammography, and introduction of adjuvant therapy. Nevertheless, breast cancer continues to be the second leading cause of cancer-related mortality in women, with estimates of 182,460 diagnosed cases and 40,480 deaths from the disease during 2008 for the United States (1).
Physical examination, together with conventional mammography, is a sensitive method for the early detection of breast cancer (2–4) and has been shown to decrease associated mortality (5,6). Mammography, however, has limitations in clinical practice; it has a low positive predictive value of 35.8% as a screening test and is moderately sensitive for detecting breast lesions (7). Also, the sensitivity for detecting breast cancer declines significantly with increasing breast density (7,8). High false-positive rates have been reported in mammography of dense breasts (9), with a sensitivity of 30%−68% in women with dense to extremely dense breasts (3,8,10). An association of breast density and breast cancer risk was first proposed by Wolfe (11), was confirmed by others (12–14), and led to the acceptance of breast density as an independent risk factor for breast cancer.
Other imaging modalities, such as CT and MRI, have a high sensitivity but low specificity in diagnosing breast cancer (15). MRI is less specific than conventional scintimammography used for the detection of nonpalpable breast lesions, and a recent meta-analysis (16) suggests that scintimammography may be a useful adjunct to mammography and physical examination in the diagnosis of breast cancer.
18F-FDG PET provides a high contrast between normal and malignant tissues because malignant tissue is hypermetabolic. This is a major advantage and makes this technique particularly valuable in the evaluation of dense breasts for malignancy, because breast density has been shown to decrease the sensitivity of mammography.
18F-FDG PET has been shown to be highly accurate in characterizing palpable breast lesions and superior to other modalities in detecting locoregional spread and distant metastasis (17–22). As with mammography, higher 18F-FDG uptake in normal breast tissue can affect the accuracy of 18F-FDG PET in the detection of breast cancer.
To our knowledge, there is little information in the literature for assessing factors that affect 18F-FDG uptake in normal breast tissue such as age, menopausal state, and the mammographic density in a systematic manner. Variation in age and breast density over time are important factors to consider (23,24) when screening and monitoring parenchymal changes with prophylactic and therapeutic interventions. Therefore, we prospectively and systematically investigated the effect of age, menopausal status, and mammographic density on 18F-FDG uptake in normal glandular breast tissue.
MATERIALS AND METHODS
Patient Population
We conducted at the University of Pennsylvania a prospective analysis of the role of 18F-FDG PET in the identification of breast cancer. This prospective National Institutes of Health (NIH)–funded program was designed to test the role of various radiologic modalities including 18F-FDG PET for the detection and staging of primary breast cancer. For the purpose of investigating age-related changes and the effect of density and menopausal state, we included women with normal contralateral breasts, excluding those with suggestive findings on MRI, ultrasonography, or digital mammography in the contralateral breast.
Two hundred fifty patients with breast cancer newly diagnosed on film-screening mammography, ultrasonography, MRI, and biopsy of the breast lesions were included in this study. This study was compliant with the Health Insurance Portability and Accountability Act and received Institutional Review Board approval. Informed consent was obtained from the patients who were enrolled in this study. No participant received chemotherapy or radiation therapy before undergoing PET scans for preoperative staging.
18F-FDG PET
Patients fasted for at least 4 h before the PET scan and had blood glucose levels less than 140 mg/dL at the time of injection. PET was initiated approximately 60 ± 2 min after intravenous administration of 18F-FDG (5.2 MBq/kg of body weight) through an indwelling catheter inserted into an antecubital vein. PET was obtained as a whole-body image, which included the entire trunk (from the neck to the groin) on all patients, using a dedicated whole-body PET scanner (Allegro; Philips Medical System). Using a 137Cs point source, we obtained transmission scans to provide attenuation correction. The images were reconstructed using an iterative reconstruction algorithm. The ordered-subsets expectation maximization method was used to reconstruct the images for clinical and research analyses (25).
Image Analysis
After image reconstruction, a region of interest (ROI) was carefully drawn on the normal breast around the glandular breast tissue as determined by visual inspection on the consequent 4–6 PET scan slices. From these ROIs, the standardized uptake value (SUV) was calculated according to the following formula: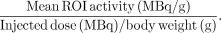
The maximum SUV (SUVmax) and the average SUV (SUVavg) of 18F-FDG of the normal glandular breast tissue were measured from the ROI, which was placed around the normal dense tissue in the breast as visualized on the PET scan slice. The intended SUVmax and SUVavg were generated from the image plane that revealed the highest SUV. The nipple and areola area were excluded from ROI placement. Two experienced nuclear medicine physicians independently measured SUV.
Breast density for all patients was classified according to mammography categories into 1 of 4 groups as defined by the Breast Imaging Reporting and Data System: almost entirely fatty (group 1), scattered fibroglandular tissue (primarily fatty) (group 2), heterogeneously dense (group 3), and extremely dense (group 4). Because we had few numbers of entirely fatty and extremely dense breasts, we combined groups 1 and 2 as a nondense breast group and combined groups 3 and 4 as a dense breast group. Also, patients were categorized into 2 groups according to their menopausal state: premenopausal or postmenopausal. We then compared and analyzed SUVmax and SUVavg of the right and left breasts to determine whether there was a difference between 18F-FDG uptake of the right and left breasts.
Statistical Analyses
The data were summarized using descriptive statistics. A Student t test of independent samples was used to compare mean differences of age between dense and nondense breasts. An analysis of covariance appropriate for a 2-factorial design was applied to assess the effects of breast density and menopausal state on SUVmax and SUVavg, adjusting for covariate age. After the significant linear effect of age on the uptake values was determined, separate simple linear regression analyses were performed to further examine the relationship between age and uptake values.
Pearson χ2 was used to test the association between menopausal state and density. Unless otherwise stated, all tests were conducted using a type I error rate (α) of 0.05. Because of missing observations, the number of cases included in different tests varied. All analyses were performed using SPSS (version 13.0; SPSS, Inc.) statistical software.
RESULTS
Of the 250 patients who were enrolled this study, 101 patients who had suggestive findings on radiologic examinations in the contralateral breasts were excluded from the analyses. Mean, SD, and minimum and maximum age for the 149 patients with confirmed normal breasts were 50.9, 9.7, and 32 and 77 y, respectively. Estimates for SUVmax were 1.16, 0.33, 0.50, and 2.30, respectively, and estimates for SUVavg were 0.67, 0.20, 0.30, and 1.20, respectively.
Breast Density, Menopausal State, and Age Relationships
Mean and SD of ages of the 92 patients with dense breasts were 49.0 ± 8.67 y, and those of the 57 patients with nondense breasts were 54.0 ± 10.51 y. The average age of patients with nondense breasts was significantly higher than that of patients with dense breasts (P < 0.01). Similarly, mean and SD of ages of the 81 premenopausal patients were 44.2 and 5.7 y, and estimates of the 68 postmenopausal patients were 58.8 and 7.1 y. Average age of postmenopausal patients was significantly higher (P < 0.01) than that of premenopausal patients.
As the result of the relationships between age and menopausal state, breast density related to menopausal state (P < 0.05).
Relationship of SUV to Breast Density, Age, and Menopausal State
Because breast density, age, and menopausal state were related, analyses of covariance appropriate for a 2-factorial design were applied to maximum and SUVavg classified by density and menopausal states and adjusted for covariate age. Analysis of covariance and least squares means, corrected for age differences, are given in Tables 1 and 2, respectively, for SUVmax, and in Tables 3 and 4, respectively, for SUVavg.
Analysis of Covariance of SUVmax
Means of SUVmax for Different Menopausal States and Densities
Analysis of Covariance of SUVavg
Means of SUVavg for Different Menopausal States and Densities
The analysis of covariance indicated that the effect of density and the linear effect of age were significant on SUVmax. Dense breasts had an average of 1.243 SUVmax, and nondense breasts had only 0.997 SUVmax. After removing the linear effect of age, menopausal state had no effect on SUVmax. Again, after removing the linear effect of age, the interaction effect of breast density and menopausal state was not significant, indicating the independence of their effect on SUVmax.
Linear effect of age on SUVmax was further investigated using linear regression analysis. Simple linear regression analysis estimates that average SUVmax decreases 0.011 units each year (P < 0.01) (Fig. 1).
Simple linear regression of SUVmax with age.
The analysis of covariance indicated that breast density and linear effect of age significantly affected SUVavg. Dense breasts had a mean of 0.694 for SUVavg, and SUVavg for nondense breasts was 0.592. After removing the linear effect of age, menopausal state had a nonsignificant effect on SUVavg. Again, after removal of the linear effect of age, the interaction effect of breast density and menopausal state was not significant, indicating their independence on SUVavg. Linear effect of age on SUVavg was further investigated using linear regression analysis. Simple linear regression analysis has shown that, on the average, SUVmax decreases 0.008 units each year (P < 0.01) (Fig. 2).
Simple linear regression of SUVavg with age.
There was no disagreement in the SUVmax calculated by the 2 investigators. However, there were minor differences—resolved by consensus between the 2 operators—with regard to SUVavg. There was no difference between 18F-FDG uptakes of right and left breasts of the different density groups.
DISCUSSION
We have published the preliminary results of this study in a review article (26). We have investigated the relationships between 18F-FDG uptake and age, breast density, and menopausal state. Vranjesevic et al. (27) retrospectively analyzed the effects of breast density on 18F-FDG uptake in 45 women with normal breast tissue. Theirs was the first published report showing that breast density affects the uptake of 18F-FDG and that the SUVs are significantly higher in dense breasts than in fatty breasts. We concur with their findings; however, our findings on the effect of menopausal state on SUV were contrary to their findings. Vranjesevic et al. (27) observed that hormonal status was a predictive variable in their stepwise multiple regression analyses, whereas our covariance analyses, using age as the covariate and after the elimination of the linear effect of age, revealed that menopausal state alone had no effect on SUV. The difference in conclusions could have been the result of the relationship between age and menopausal state. Once the linear effect of age is removed, as is done in our covariance analysis, there might be little variability for the menopausal state to explain.
In another study (28), the authors reported that age and menopausal status had not emerged as significant predictors for 18F-FDG uptake using stepwise multiple regression analyses. In our study, age was a statistically significant predictor for SUV, perhaps because of the differences in the sizes of samples (96 vs. 149 in our study). Their results regarding the effect of breast density were in line with previously published and present studies (27,29,30).
The results of our analyses showed that the average age of patients with nondense breasts was significantly higher than that of patients with dense breasts (P < 0.01) (Fig. 3). The average age of postmenopausal patients was significantly higher than that of premenopausal patients. As the result of the relationships between age and menopausal state, qualitative breast density related to the menopausal state (P < 0.05). After removal of the linear effect of age, menopausal state had no effect on SUVmax and SUVavg. In other words, age and qualitative breast density are independent factors and significantly affect 18F-FDG uptake for both SUVmax and SUVavg.
(A) Axial and sagittal images of subject with mammographically nondense breasts. Measured SUVmax and SUVavg of glandular tissue were 0.8 and 0.5, respectively. (B) Axial and sagittal images of subject with mammographically dense breasts. Measured SUVmax and SUVavg of breast are 1.4 and 1.0, respectively. Difference between degrees of 18F-FDG uptake is also visually apparent on these images.
Our data demonstrated that there is a significant decrease of 18F-FDG uptake as age increases. To our knowledge, this is the first study that shows a statistically significant correlation between age and 18F-FDG uptake in the breast tissue. The radiographic data in our study population further confirmed those of previous studies in the mammography literature, which showed older women tend to have fatty breasts. Younger women tend to have denser breasts than do older women (31–34). Therefore, we were able to demonstrate that the 18F-FDG uptake in the breast decreases with age, contradicting the results of the study by Vranjesevic et al. (27). In addition, qualitative breast density is considered an independent risk factor for breast cancer (11–13). We have recently presented (35) our investigation about the effect of qualitative breast density on washing out of 18F-FDG from normal breast tissue. According to this other report, the SUVmax and SUVavg results between the 2 groups (dense and nondense breasts) were statistically significant, but the percentage change over time was not. To our knowledge, this is also the first report of dual-time-point changes in normal breast tissue regarding density. The change of 18F-FDG uptake does not depend on the density of the breast. This finding is especially important for malignancies in dense breasts, in which the 18F-FDG uptake in the tumor increases with time, whereas uptake decreases in normal breast tissue regardless of density, improving the contrast resolution between the tumor and the surrounding background activities.
According to our results in the present study, menopausal state has no effect on 18F-FDG uptake after removal of the linear effect of age. In the study by Vranjesevic et al. (27), the authors reported that the breasts of premenopausal women had a higher SUV than did the breasts of postmenopausal women not receiving hormonal therapy. In contrast, postmenopausal women receiving hormonal therapy had SUVs similar to those of the premenopausal women. Thus, the authors concluded that hormonal therapy in postmenopausal women appears to normalize the glucose metabolic activity of normal breast tissue. They found these results by analyzing 36 subjects, 12 in each category. In our study, we had no patients who were receiving hormonal therapy, so we could not confirm these findings. However, in the literature (36,37), hormone replacement therapy or hormonal stimuli has been shown to increase qualitative breast density. Therefore, we believe that further studies are necessary with postmenopausal patients who are on hormone therapy to determine the combined effect of age and this therapeutic intervention.
An additional different result in the study by Vranjesevic et al. (27) was that 18F-FDG uptake was slightly, but significantly, higher in the right breast than in the left breast. On average, they found that the metabolic activity in the left breast was about 10% lower than that in the right breast. Our study of 149 subjects showed no significant difference in 18F-FDG uptake between right and left breasts.
In our study and that of Vranjesevic et al. (27), the measurements were stable and the interobserver variability was low, emphasizing the excellent reproducibility.
We did not adjust the SUVs for lean body mass or total body surface area because the corrected and uncorrected SUVs were shown to be highly correlated by a regression analysis in the study by Vranjesevic et al. (27). Thus, there is likely no need to correct for lean body mass when analyzing normal breast tissue, because the 18F-FDG SUVavg in these tissues generally is below 1.0.
CONCLUSION
Our data clearly demonstrated that the age of the subject and the density of the breast are independent and important factors in 18F-FDG uptake in normal breast tissue. There was a statistically significant decrease in 18F-FDG uptake as the age increased. Although the younger, premenopausal patients or the patients with dense breasts have higher SUVs, the accuracy of 18F-FDG PET studies in diagnosing malignant breast tumors may not be significantly affected because of the high contrast between the tumor and the surrounding uptake of this radiotracer. Our findings represent functional data regarding changes in breast tissue metabolic activity with aging—data that may become important as imaging techniques are used to monitor new prophylactic and therapeutic interventions including novel hormonal and pharmacologic entities.
Acknowledgments
This study was supported by the NIH Public Health Services research grant PO1-CA85424.
Footnotes
-
COPYRIGHT © 2010 by the Society of Nuclear Medicine, Inc.
References
- Received for publication July 23, 2009.
- Accepted for publication December 3, 2009.










