Abstract
Recent clinical and experimental data demonstrate that thyroid stunning is caused by previous irradiation and may influence the efficacy of 131I radiation therapy of thyroid cancer and possibly hyperthyroidism. To avoid stunning, many clinics have exchanged 131I for 123I for pretherapeutic diagnostic imaging and dose planning. Furthermore, recent in vitro studies indicate that 131I irradiation reduces iodide uptake by downregulating the expression of the sodium iodide symporter (NIS). The rationale for this study was therefore to study effects on iodide transport and NIS messenger RNA (mRNA) expression in thyrocytes exposed to both 123I and 131I in addition to some other potentially interesting radionuclides. Methods: Thyrotropin-stimulated thyroid cell monolayers were exposed to 0.5 Gy of 123I, 131I, 99mTc, or 211At, all being radionuclides transported via NIS, in the culture medium for 6 h, or to various absorbed doses of 123I or 131I for 48 h. NIS mRNA expression was analyzed using quantitative reverse-transcriptase polymerase chain reaction. Results: Iodide transport and NIS mRNA expression were reduced by all radionuclides. At the same absorbed dose, iodide transport was reduced the most by 211At, followed by 123I and 99mTc (equally potent), whereas 131I was least effective. The onset of NIS downregulation was rapid (<1 d after irradiation) in cells exposed to 123I or 211At and was delayed in cells irradiated with 131I or 99mTc. Iodide transport and NIS expression were recovered only for 211At. 123I reduced the iodine transport and the NIS mRNA expression more efficiently than did 131I at an equivalent absorbed dose, with a relative biological effectiveness of about 5. Conclusion: The stunning effect per unit absorbed dose is more severe for 123I than for 131I. Despite the lower absorbed dose per unit activity for 123I than for 131I, stunning by 123I cannot be excluded in patients. The degree to which iodide transport capacity and NIS mRNA expression are reduced seems to be related to the biological effectiveness of the type of radiation delivering the absorbed dose to the target, with 211At (which has the highest relative biological effectiveness) causing the highest degree of stunning per unit absorbed dose in the present study.
Thyroid stunning is a complication of radiation therapy with 131I, the consequences of which affect mainly the management of thyroid carcinoma patients (1–6). The stunning phenomenon is characterized by less 131I uptake at therapy than was predicted from the 131I uptake measurement during dose planning. The reason for this effect is not fully understood, although several mechanisms have been proposed. One explanation, rejecting an effect from the diagnostic 131I, is that a low 131I content measured in treated tumors reflects a rapid loss of viable tissues and that dying cells damaged by irradiation are incapable of keeping accumulated iodide (7,8). In this case, thyroid stunning would be regarded as an artifact due to an early therapeutic effect of the ablative 131I and hence not a clinical problem. Another suggested mechanism originates from the hypothesis that irradiation might disturb the uptake of iodide per se independently of radiation effects on vital cell functions and thus before cell viability eventually is affected. This possibility is supported by experimental studies showing that thyrotropin-stimulated iodide transport mediated by the sodium iodide symporter (NIS) is markedly reduced when cultured thyroid cells are irradiated with relatively small 131I doses (0.15–3 Gy) equivalent to those estimated to be received after diagnostic administration of 131I in vivo (9,10). Moreover, 131I irradiation reduces expression of NIS at the transcriptional level in still-viable thyrocytes (11). Together, this suggests that stunning may result from a decreased synthesis of NIS leading to diminished amounts of iodide-transporting protein in preirradiated cells.
Thyroid stunning potentially threatens the effectiveness of a given ablative amount of 131I. To avoid this problem, 123I has been proposed to replace 131I for diagnostic uptake measurements. Stunning has nevertheless occurred in thyroid remnants and metastases after administration of 123I within a rather wide range of diagnostic activities (50–200 MBq) (7,12,13). However, whether 123I irradiation triggers cellular changes leading to loss of NIS expression and reduced iodide uptake has not been experimentally investigated. Likewise, it is not known if other radionuclides such as 211At and 99mTc, which also accumulate in the thyroid by NIS-mediated uptake (14,15), may cause stunning. 99mTcO4− (pertechnetate) is routinely used in the functional evaluation of thyroid nodules and goiters. 211At is an α-emitter suggested for therapy of poorly differentiated thyroid carcinoma (16,17) and nonthyroid tumors expressing NIS after gene transfer (18).
99mTc, 211At, 123I, and 131I have different decay properties. The main part of the absorbed dose is deposited by particles, but the type of particle, the energy, and the yield differ considerably among these radionuclides. For example, α-particles and Auger electrons characterized by high-linear-energy transfer and relative biological effectiveness (RBE) (19) are predominantly emitted by 211At and 123I, respectively. Thus, comparison of these nuclides to see which type of radiation is more prone to inducing stunning would be of interest. In this study, we therefore investigated first whether 123I, 99mTc, and 211At are able to induce stunning in vitro and second whether iodide transport and NIS messenger RNA (mRNA) expression might be affected differently by 123I, 99mTc, and 211At irradiation than by 131I irradiation analyzed at equivalent absorbed doses. To mimic internal radiation of the normal thyroid in vivo, quiescent (G0) primary thyroid cells cultured as a monolayer in bicameral chambers were continuously irradiated for 6 or 48 h while the nuclides were transported across the epithelium and gradually accumulated in the culture compartment corresponding to the follicular lumen.
MATERIALS AND METHODS
Cell Culture
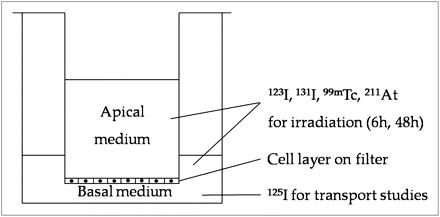
Porcine thyroid primary cells, prepared as previously described (9), were plated on collagen-coated micropore filters (pore size, 0.4 μm) in bicameral chambers (Transwell, 3413; Corning Costar) and cultured for 7 d at 37°C in an incubator with 5% CO2 in Earle minimum essential medium supplemented with 5% fetal calf serum, penicillin (200 U/mL), streptomycin (200 μg/mL), and amphotericin B (2.5 μg/mL) (PAA Laboratories GMbH). The medium in the culture chamber was replaced every 2–3 d. At confluency, the growth of the proliferating cells was arrested and they established a tight monolayer, of which the barrier function was monitored by measuring transepithelial resistance with a Millicell-ERS ohmmeter (Millipore Corp.). The basal and apical compartments of the bicameral chamber correspond to the extrafollicular space and the follicular lumen, respectively (Fig. 1). All experiments were thus performed on confluent cells, of which most were postmitotic.
Transwell bicameral culture chamber system. Pig thyrocytes were grown on microporous filter (pore size, 0.4 μm) that divides well into apical and basal compartments corresponding to follicular lumen and extrafollicular space, respectively. Cells were irradiated with 123I, 131I, 99mTc, or 211At in medium, both apically and basally. Gradual accumulation of radionuclide in apical culture medium due to NIS-mediated transport in basal-to-apical direction was taking place during irradiation. After irradiation, cell cultures were analyzed for 125I− transport capacity (125I was added basally), total DNA content, and NIS mRNA expression.
Radionuclides
All iodine isotopes used (123I, 125I, and 131I) were obtained as NaI (Nycomed Amersham, PLC). 99mTc was obtained as pertechnetate, 99mTcO4−, by elution of a 99Mo/99mTc generator (UltraTechnekow FM; Mallinckrodt Medical). 211At was produced at the Cyclotron and PET Unit at Rigshospitalet, Copenhagen, Denmark, by the 209Bi(α,2n)211At reaction, where the targets, aluminum backings with 19- to 24-μm 209Bi layers, were prepared at the Department of Physics, Chalmers University of Technology, Göteborg, Sweden. 211At was distilled according to methods previously described (20). The physical properties of the radionuclides are given in Table 1.
Physical Properties of Radionuclides Studied (22)
Absorbed Dose Calculations
Estimations of the absorbed dose to the cell layer from 131I, 123I, or 99mTc were based on Monte Carlo simulations with the PENELOPE code and PENCYL program (21), as described earlier (10). Radionuclide decay data were collected from MIRD: Radionuclide Data and Decay Schemes (22). The absorbed dose for 211At was calculated using the time integral of equilibrium dose rate from 211At in the basal and apical compartments. The increasing activity concentrations in the apical medium generated by ongoing transepithelial (from basal to apical) transport of the radionuclides during irradiation was taken into consideration.
Irradiation Procedures
Before irradiation, cell cultures were stimulated with thyrotropin (1 mU/mL; Sigma-Aldrich Sweden AB) for 48 h to upregulate the NIS expression and iodide transport capacity. Thyrotropin stimulation of cell cultures was maintained until iodide transport was evaluated.
Irradiation was performed mainly in accordance with the protocols of earlier studies (9–11). Two types of experiments were investigated: a comparison of the effects of a standardized dose (0.5 Gy) from 99mTc, 123I, 131I, or 211At, and a comparison of the dose–response relationship between 123I and 131I irradiation. In the first setup, culture medium containing 99mTc (9.7 MBq/mL), 123I (7.8 MBq/mL), 131I (0.63 MBq/mL), or 211At (22 kBq/mL) was added to the bicameral chambers, both apically (200 μL) and basally (400 μL) (Fig. 1). The mean absorbed dose to the cells was 0.5 Gy after 6 h of exposure. The short exposure time was chosen to keep the change in dose rate low for the short-lived radionuclides 99mTc and 211At (physical half-life, 6.0 and 7.2 h, respectively). In the second setup, cell cultures were irradiated with various activity concentrations of 123I (0.034–40 MBq/mL) and 131I (0.20–2.0 MBq/mL) for 48 h to mimic the longer exposure assumed for the thyroid in vivo. The activity of the medium added apically (100 μL) and basally (500 μL) resulted in an absorbed dose to the cell layer of 0.01–14 Gy and 2.9–14 Gy for 123I and 131I, respectively.
Organification of radioiodine was prevented by adding methimazole (1 mmol/L; Sigma-Aldrich Sweden AB) to the culture medium in all radiation experiments. Culture plates, one for each radionuclide and absorbed dose, were kept shielded by lead in the CO2 incubator at 37°C to prevent external cross irradiation between groups. Accidentally damaged or leaky cultures unable to concentrate radionuclide in the apical culture compartment during ongoing irradiation were identified by medium sampling and counting of activity content in the apical and the basal medium after exposure and were excluded. Irradiation was stopped by washing the cells in radionuclide-free medium at least 3 times before further culturing. None of the radionuclide used for irradiation of the cell culture remained after washing.
125I− Transport Studies
The transepithelial iodide transport capacity in irradiated and corresponding control cultures was evaluated using 125I− as tracer. The basal medium was replaced with medium containing 125I− (60 kBq/mL), and after transport had been allowed for 30 min at 37°C, 50-μL samples were taken from both the apical and the basal compartments. The activity in the samples was measured with a γ-counter (Wallac 1480 WIZARD 3"; Wallac Oy). Corrections were made, when necessary, for radioactive decay and background. The relative 125I− transport through the cell layer, defined as the ratio between the amount of 125I− transported by the irradiated cell cultures and that transported by the nonirradiated control cells, was determined. The same cultures were subjected to repeated 125I− transport studies to monitor iodide transport changes over time. After each transport study, the cell cultures were washed with fresh medium containing no radionuclides and then reincubated until the next transport study. The repeated short exposure to 125I−, for about 30 min, in each transport measurement did not affect the basal transport rate as compared with that measured in matching nonirradiated cells (data not shown). The experiments were reproducible, and each group consisted of 3–6 cell cultures.
Quantitative Reverse-Transcriptase Polymerase Chain Reaction (qRT-PCR) Analysis of NIS mRNA Expression
NIS transcript levels were quantified by qRT-PCR using a previously established protocol (11). Total RNA was extracted from the cell cultures using the RNeasy Micro Kit (Qiagen GmbH). The RNA was quantified with spectrophotometry at 260 nm, and 0.5 μg was used for each sample to synthesize complementary DNA with random hexamers and TaqMan Reverse Transcription Reagents (Applied Biosystems). Primers to the porcine NIS gene and 18S (reference gene) were designed with the Primer Express Software (Applied Biosystems) according to the following templates: porcine NIS forward primer was 5′-ctctcctggcagggcatatct-3′; porcine NIS reverse primer was 5′-gctgagggtgccgctgta-3′; 18S forward primer was 5′gtaacccgttgaacccatt-3′; and 18S reverse primer was 5′-ccatccaatcggtagtagcg-3′ (TAG Copenhagen A/S). The relative amounts of polymerase chain reaction products were quantified using QuantiTect SYBR Green (Promega) and the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). The thermal cycling conditions were an initial cycle of 2 min at 50°C, a 15-min cycle at 95°C, 40 cycles of 15 s each at 94°C (denaturation), and a cycle of 1 min at 60°C (annealing and extension). All amplification reactions were done in triplicate. The threshold cycle values were used for calculation of the relative RNA expression ratios between control and treated cell samples, according to Pfaffl (23).
Cell Number Quantification
To rule out the possibility that any findings were related to altered cell number (i.e., due to loss of radiation-damaged cells), the total DNA content of cultures exposed to radionuclides was measured using a fluorometric DNA assay (24). The viability of the cells was monitored by iodide transport studies.
Statistical Analysis
Results from iodide transport studies are given as mean ± SEM in the figures. The Student t test was used to determine the statistical significance of differences between data obtained from the experimental groups. P < 0.05 was considered significant. NIS mRNA data from qRT-PCR analyses were evaluated with the relative expression software tool (23). P < 0.001 was considered statistically significant.
RESULTS
Thyrotropin-stimulated thyroid cell monolayers were irradiated with 123I, 131I, 99mTc, or 211At in the culture medium for 6 h, resulting in a standardized absorbed dose of 0.5 Gy. During exposure, the radionuclides were actively transported across the cell layer resulting in different apical-to-basal activity concentration ratios at the end of irradiation (6.0 for 125I, adopted for 123I and 131I; 8.5 for 211At; and 14 for 99mTc). These results were considered in the dosimetric calculations.
As shown in Figure 2, the transepithelial transport of iodide (monitored by 125I−) was reduced by all radionuclides studied. In general, the transport started to decrease 1–2 d after irradiation and was suppressed most significantly after 5–7 d. Immediately after irradiation, a higher transport capacity was shown by the cell cultures than by nonirradiated control cells. This was, however, statistically significant only for 211At-irradiated cultures. At the given absorbed dose (0.5 Gy), the strongest inhibitory effect on iodide transport, amounting to nearly 70% of the control level, was observed in 211At-irradiated cells. 123I and 99mTc were equally potent and decreased iodide transport by 30%−40%, whereas 131I-irradiated cells reduced transport by less than 20%. Interestingly, whereas no significant recovery from stunning was evident in cultures exposed to 131I, 123I, or 99mTc, iodide transport was statistically significantly increased between 5 and 7 d in cells that had been irradiated with 211At (Fig. 2).
Time-dependent iodide (125I−) transport changes in filter-cultured thyrocytes after irradiation with 123I, 131I, 99mTc, or 211At to 0.5 Gy for 6 h. Effects of irradiation are presented relative to transport level monitored in nonirradiated cultures at each time point. Results are given as mean ± SEM (n = 6). *Statistically significant difference from controls, P < 0.05.
The expression of NIS mRNA was investigated in cell cultures that were irradiated in parallel with those analyzed for changes in 125I− transport. Similar to the effects on iodide transport, the smallest and largest reduction of NIS transcript levels were evident in cells exposed to 131I and 211At, respectively. However, several additional differences were observed. Most strikingly, 123I and 211At had already suppressed the NIS expression 24 h after irradiation, whereas the suppression was delayed in cells exposed to 131I and 99mTc. Moreover, the NIS transcript level recovered completely in cells exposed to 123I and partially in cells exposed to 211At after culture for 5 d after irradiation (Fig. 3 and Table 2). NIS expression did not recover in cells exposed to 131I or 99mTc during the interval studied.
Changes of NIS mRNA expression in thyroid cells irradiated with 123I, 131I, 99mTc, or 211At at same dose (0.5 Gy) and exposure times as shown in Figure 2 (representing data from parallel cultures in same experiments) on days 1 and 5 after irradiation. qRT-PCR data are presented as log2 expression levels compared with those of matched nonirradiated controls (n = 3). *Statistically significant difference from controls, P < 0.001.
Changes of NIS mRNA Expression in Thyroid Cells Irradiated with 123I, 131I, 99mTc, or 211At to Absorbed Dose of 0.5 Gy During 6 Hours, on Days 1 and 5 After Irradiation
The effects of 131I and 123I on thyrotropin-stimulated iodide transport were compared in dose-response experiments after irradiation for 48 h (Fig. 4). 131I reduced the transepithelial 125I− transport, in an absorbed dose-dependent manner, by approximately 30% at 2.7 Gy and 85% at 13 Gy (Fig. 4). Significantly lower absorbed doses of 123I were required to induce a corresponding reduction of iodide transport.
Dose-dependent iodide (125I−) transport changes in filter-cultured thyroid cells irradiated with 131I or 123I for 48 h. Effect on transport was evaluated 3 d after end of radionuclide exposure. Results obtained from 3 separate experiments, with triplicates in each, are given relative to matching nonirradiated controls as mean ± SEM (n = 3). Inset shows details at low absorbed doses.
All experiments were performed on confluent and growth-arrested (i.e., G0) cells. There were no significant changes in DNA content between nonirradiated and irradiated cultures either immediately or 5 d after radionuclide exposure (data not shown), indicating that the total cell number was not influenced by the irradiation.
DISCUSSION
Four radionuclides, 123I, 131I, 99mTc, and 211At, known to be concentrated in the thyroid by NIS-mediated transport were compared for their ability to induce thyroid stunning in vitro at a standardized exposure time (6 h) and absorbed dose (0.5 Gy). All were found to inhibit iodide transport in cultured thyroid cells 1–5 d after the irradiation. The potency to induce stunning differed significantly (131I < 99mTc = 123I < 211At), ranging from 20%−80% transport inhibition (Fig. 2). This difference was evident also when the maximal downregulation of NIS expression was compared by qRT-PCR analysis (Fig. 3). Interestingly, the decrease in NIS transcription was much faster in cells irradiated with 211At or 123I, already appearing after 1 d, than in cells exposed to 99mTc or 131I. The response to 211At and 123I also differed from the response to the other 2 radionuclides in that NIS expression and iodide transport partly recovered within 5 d after irradiation. Decreased expression of NIS at the transcriptional level seems to be a common mechanism leading to inhibited iodide transport. However, the magnitude and kinetics of radiation-induced stunning differ between the radionuclides studied, probably because of the different physical properties of the radionuclides (Table 1).
RBE is defined as the ratio of the absorbed doses, from a reference radiation type (usually photons) and a test radiation that causes equal biologic effect (19). Low-energy electrons (E ≤ 50 keV) such as Auger electrons and α-particles ionize matter more densely than do photons or conventional electrons with higher energy, resulting in higher RBE. Auger electrons have RBE values of 1.5–40 depending on the electron energy and the location of the radionuclide, because of the limited range of these particles (25). Likewise, reported RBE values for the α-particle emitter 211At vary between 1.5 and 25 for different tissues, experimental conditions, and endpoints (26–33). In the present study, the dose-response curves presented in Figure 4 allow an estimation of RBE (at the absorbed dose levels required to reduce the relative 125I transport by 1 natural log, 37%) for the stunning effect induced by 123I, compared with 131I, which was about 5 (estimated from Fig. 4). RBE for stunning could, however, not be determined for the other radionuclides according to the strict definition. However, at 5 d after irradiation (Fig. 2), which probably is close to the time at which the maximal inhibitory effects of the nuclides on iodide transport are monitored, 211At was 2.4 times more efficient than 131I and 1.7 times more efficient than 99mTc in inducing stunning.
99mTc and 123I emit relatively low-energy electrons that are responsible for most of the absorbed dose to the cells, compared with 131I, for which more than 90% of the absorbed dose is delivered by higher-energy electrons (Table 1). The effectiveness of 99mTc and 123I in causing stunning was similar, although NIS expression was downregulated more quickly by 123I than by 99mTc. It is reasonable to assume that the relatively poor stunning effect of 131I, compared with 123I or 99mTc, depends on the fact that the latter have a higher abundance of low-energy electrons (0–10 keV) that might give rise to more complex DNA lesions. This explanation requires that the radionuclide be transported closely to the cell nucleus.
The different dose rates from the investigated nuclides might also influence the cellular responses to irradiation. 99mTc and 211At had initially the highest dose rate, their half-life being close to the total exposure time, resulting in about a 50% reduction in dose rate during exposure. In contrast, the long half-life of 131I made the dose rate relatively stable during the irradiation period. The higher dose rate can thus be responsible at least in part for the fast downregulation of the NIS-mRNA expression in cells irradiated with 211At or 123I. The relatively slow response to 99mTc must, however, have another explanation. 99mTc was transported twice as quickly across the epithelial cell layer as the other radionuclides. The reason for this difference is unknown, although previous studies comparing transport of 125I− and 211At suggest different transport properties between radionuclides concentrated in thyroid cells (14,34). A short intracellular transit time decreases the probability that low-energy electrons from 99mTc reach the cell nucleus, eventually leading to less or delayed interference with the transcriptional machinery regulating NIS. In addition, a lower emission yield of electrons in the range of 20–40 keV might further weaken the transcriptional response to 99mTc relative to that of 123I.
The decrease in NIS mRNA levels was rapid, preceding the earliest signs of inhibited iodide transport, in 211At- and 123I-irradiated cells. We have previously shown that loss of iodide transport accompanies downregulation of NIS also in cells irradiated with 131I at a higher dose (7.5 Gy) than used here (11). Although other mechanisms, that is, posttranscriptional, may contribute, the suggestion is strong that reduced transcriptional activity of the NIS promoter is a key feature leading to stunning in normal thyroid cells. Notably, the half-time of native NIS protein—estimated at 3 d in thyrotropin-starved cells and 5 d after thyrotropin stimulation—is unusually long (35). The slow turnover of NIS probably reflects a mechanism by which newly synthesized NIS is retained in the plasma membrane. Such a mechanism might be of physiologic importance in keeping a high iodide uptake capacity although other thyroid functions may fluctuate. It will thus take a considerable time for a reduced NIS gene expression to be translated into a decreased number of functionally active NIS molecules at the cell surface. Therefore, it is not surprising that radiation-induced loss of iodide transport develops gradually and rather slowly after the NIS gene transcription is significantly suppressed. Moreover, these in vitro findings support the many clinical reports of a delay in detection of stunning until several days after the administration of radioiodine for diagnostic purposes (1–7,36,37).
A central question not previously investigated is whether thyroid stunning is irreversible, being part of a general stress response to radiation-induced damage that eventually is lethal, or whether iodide transport may recover in the affected cell. Clinical studies indicate that stunning is a prolonged effect lasting for many days (1,3,6,7,36). This finding was confirmed in the present study, in which no signs of recovery of the suppressed iodide transport were observed in cultures exposed to 131I or 99mTc up to 1 wk after the irradiation period. It was therefore surprising that the 211At-irradiated cells regained iodide transport capacity, accompanied by a partial normalization of the NIS transcript level. As clear signs of recovery of the NIS expression also were noted in cells exposed to 123I, it is tempting to speculate that early recovery from stunning might require that the NIS downregulation be rapidly triggered by high-linear-energy-transfer radiation. This possibility further suggests, assuming that loss of NIS expression directly or indirectly is part of a transcriptional response to irradiation-induced DNA damage, that the incidence and type of DNA lesions and the efficiency with which DNA repair mechanisms are activated determine the kinetics of stunning. However, no statistically significant recovery was seen for 99mTc-irradiated cells even though the emission profile is quite similar to that of 123I. This difference may be due to the shorter transit time of 99mTc. This shorter transit time is demonstrated by the higher apical-to-basal activity concentration ratio at the end of irradiation for 99mTc (14), compared with 123I (6.0). Although the recovery mechanism is yet to be elucidated, this finding is intriguing as it indicates that stunning is not a static process but can be modulated. Moreover, because the observations were made on postmitotic cells that did not change in number during or after irradiation, it is the actual irradiated cells that recover from stunning, with little or no contribution from cell renewal.
123I has to some extent started to replace 131I for dose planning before 131I therapy in thyroid cancer, partly because its preferable photon energy results in a superior quality of scintigraphic images, but mainly because it is supposed to not cause stunning. For dose planning, an activity of 74–370 MBq of 131I is usually administered, compared with 10–200 MBq of 123I (1,6,7,13,36–39). According to Johansson et al. (40), the absorbed dose per unit activity is 490 and 5 mGy/MBq to the thyroid gland from 131I and 123I, respectively. Thus, the absorbed dose per unit activity in the thyroid is about 100 times lower from 123I than from 131I. Although this factor is smaller in smaller targets such as thyroid remnants and metastases, because of the lower absorbed fraction of the electrons from 131I, 123I would be preferable to avoid stunning. In this study, we show that iodide transport in cultured thyroid cells is reduced about twice as much by 123I as by 131I per unit of absorbed dose. This finding may explain why stunning may yet occur when 123I is used for dose planning, as recently reported in some clinical studies (7,12,13).
CONCLUSION
123I causes a more severe stunning effect per unit absorbed dose than does 131I, with an RBE value of about 5. Although the absorbed dose to the thyroid gland per unit activity is only 1/100 of that delivered by 131I, and somewhat higher in small thyroid remnants and metastases, stunning due to 123I cannot be excluded in patients. The degree of the reduction in iodide transport capacity seems to be related to the biologic effectiveness of the type of radiation delivering the absorbed dose to the target, with 211At causing the highest degree of stunning per unit absorbed dose in the present study.
Acknowledgments
We acknowledge Johanna Dalmo for supplying 131I, and we acknowledge Dr. Sture Lindegren at the Department of Radiation Physics, Göteborg University, and Dr. Holger Jensen at the PET and Cyclotron Unit at Rigshospitalet, Copenhagen, for supplying 211At. This study was supported by grants 3427 and 4567 from the Swedish Cancer Society, by the Swedish Radiation Protection Authority, by grant 537 from the Swedish Research Council, and by the King Gustav V Jubilee Clinic Cancer Research Foundation.
Footnotes
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication December 16, 2008.
- Accepted for publication March 9, 2009.