Abstract
Current investigations of cell transplant therapies in damaged myocardium are limited by the inability to quantify cell transplant survival in vivo. We describe how the labeling of cells with 111In can be used to monitor transplanted cell viability in a canine infarction model. Methods: We experimentally determined the contribution of the 111In signal associated with transplanted cell (TC) death and radiolabel leakage to the measured SPECT signal when 111In-labeled cells were transplanted into the myocardium. Three groups of experiments were performed in dogs. Radiolabel leakage was derived by labeling canine myocardium in situ with free 111In-tropolone (n = 4). To understand the contribution of extracellular 111In (e.g., after cell death), we developed a debris impulse response function (DIRF) by injecting lysed 111In-labeled cells within reperfused (n = 3) and nonreperfused (n = 5) myocardial infarcts and within normal (n = 3) canine myocardium. To assess the application of the modeling derived from these experiments, 111In-labeled cells were transplanted into infarcted myocardium (n = 4; 3.1 × 107 ± 5.4 × 106 cells). Serial SPECT images were acquired after direct epicardial injection to determine the time-dependent radiolabel clearance. Clearance kinetics were used to correct for 111In associated with viable TCs. Results: 111In clearance followed a biphasic response and was modeled as a biexponential with a short ( ) and long (
) and long (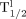 ) biologic half-life. The
) biologic half-life. The  was not significantly different between experimental groups, suggesting that initial losses were due to transplantation methodology, whereas the
was not significantly different between experimental groups, suggesting that initial losses were due to transplantation methodology, whereas the  reflected the clearance of retained 111In. DIRF had an average
reflected the clearance of retained 111In. DIRF had an average  of 19.4 ± 4.1 h, and the
of 19.4 ± 4.1 h, and the  calculated from free 111In-tropolone injected in situ was 882.7 ± 242.8 h. The measured
calculated from free 111In-tropolone injected in situ was 882.7 ± 242.8 h. The measured  for TCs was 74.3 h and was 71.2 h when corrections were applied. Conclusion: A new quantitative method to assess TC survival in myocardium using SPECT and 111In has been introduced. At the limits, method accuracy is improved if appropriate corrections are applied. In vivo 111In imaging most accurately describes cell viability half-life if
for TCs was 74.3 h and was 71.2 h when corrections were applied. Conclusion: A new quantitative method to assess TC survival in myocardium using SPECT and 111In has been introduced. At the limits, method accuracy is improved if appropriate corrections are applied. In vivo 111In imaging most accurately describes cell viability half-life if 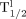 is between 20 h and 37 d.
is between 20 h and 37 d.
- 111In SPECT
- cell transplant survival
- myocardial infarction
- canine bone marrow stromal cells
- cell tracking
Although the prevalence of heart failure rises, current therapies inadequately address the pathophysiology leading to adverse myocardial tissue remodeling. Such remodeling can be activated by myocardial infarction (MI); ultimately, heart function deteriorates, initiating global dysfunction (1–5). To assess the treatment efficacy of cellular cardiomyoplasty, noninvasive and quantitative methods to track cell transplant survival in vivo are required (6). Of critical importance is improving cell survival and retention after transplantation by optimizing combinations of cell type (6,7), injection strategy (6,7), and injection time after infarction (6–9). Previous studies have demonstrated that cell delivery strategies affect the retention of cells in the myocardium (10–12). Therefore, a noninvasive method to quantify cell survival will help evaluate methods that aim to optimize survival, particularly within the first 2 wks after transplantation.
Noninvasive cell tracking technologies to determine cell survival, engraftment, and subsequent function require that existing imaging methodologies are optimized for sensitivity, specificity, and resolution of nondistributed sources. Numerous studies have imaged cell biodistribution and homing by directly labeling cells with radionuclides using SPECT and PET, which have sufficient sensitivity to detect nanomolar quantities (6) of γ-emitters in vivo. However, cellular quantification using these methods is complicated by radiolabel leakage from viable cells and clearance of radiolabeled cellular debris. Problems with significant radioisotope label leakage within the first hour have been reported with 18F-FDG (13–15), 64Cu-pyruvaldehyde bis(N4-methylthiosemicarbazone) (13), and 99mTc-hexamethylpropylene amine oxime (14). In addition to nuclear medicine techniques, MRI of contrast-labeled cells has been successful; however, sensitivity differences allow the detection of smaller amounts of contrast material using nuclear medicine techniques relative to MRI in large animal dual-labeling experiments (5).
The objective of this work was to demonstrate that 111In-tropolone can monitor transplanted cell (TC) viability in a large animal model and that cell survival after transplantation in the canine myocardium can be estimated in vivo using radiolabeled cells and SPECT. The 111In signal detected by SPECT corresponds to the sum of activity remaining within transplanted viable cells, released from dead cells, and leaked from viable cells. To correlate 111In activity to viable cells, we hypothesized that by modeling the time-dependent clearance of 111In at the injection site, we could remove the confounding radioactive signal attributed to activity within the volume of interest (VOI) but not contained within viable cells. Radiolabel leakage rate and clearance associated with transplanted cell death were acquired in vivo; these data were used to develop a corrected viable cell activity curve, demonstrating that 111In cell labeling before myocardial transplantation can accurately estimate cell survival half-life between 20 h and 37 d.
MATERIALS AND METHODS
Stromal Cell Culture and Labeling
Canine studies were approved by the Animal Use Subcommittee at the University of Western Ontario. Three weeks before surgery, bone marrow was aspirated (10–15 mL) from the humerus of female mongrel dogs (20–25 kg; 1–2 y) after anesthesia was administered. Bone marrow mononuclear cells (BMMNCs) were isolated (Ficoll-Hypaque, 1.077 g/mL; Sigma) and plated in Dulbecco's Modified Eagle's Medium (DMEM) (Invitrogen) containing 10% fetal bovine serum and antibiotics (100 units of penicillin per milliliter and 100 μg of streptomycin per milliliter; Invitrogen). Cells were subsequently incubated (37°C, 5% CO2) and floating cells were progressively washed away, leaving the adherent bone marrow stromal cell (BMSC) population that was subsequently culture-expanded for 3 wks. 111In-tropolone cell labeling has been described previously (16). Briefly, BMSCs were incubated with 111In-tropolone for 30 min at 37°C, and incubation was stopped by the resuspension of cells in 25 mL of DMEM, followed by centrifugation (430g for 5 min) and supernatant removal. Cells were washed again with phosphate-buffered saline (PBS), and the cell pellet was collected after centrifugation. The cell pellet (A) and supernatant including washes (B) were counted for 111In activity using a dose calibrator (CRC-12; Capintec) to determine labeling efficiency (A/[A + B] × 100%).
Surgical Preparation
After anesthesia, a left thoracotomy was performed, exposing the anterior surface of the heart. In 12 of 19 dogs, MI was induced by placing a snare ligature around the left anterior descending coronary artery (LAD) distal to the first diagonal branch. After 2–3 h of occlusion, the LAD was either reperfused for 2–3 h by releasing the snare before injection or remained permanently occluded. Radiolabeled injections were performed as outlined below. Within 30–40 min of injection, the incision was closed and the animal was moved to the SPECT suite.
In Vivo Experiments
To determine the contributions of cell death, radiolabel leakage, and cell survival to the 111In signal detected, 3 different in vivo experiments were performed.
To determine the contribution from cellular debris, approximately 1 × 107 BMMNCs (n = 9) or BMSCs (n = 2) were labeled in vitro with 111In-tropolone and subjected to ultrasonication (Sonic Dismembrator 500; Fisher Scientific) continuously for 5 min to cause cell lysis. The cellular debris was injected into normal (n = 3) or infarcted canine myocardium that was either permanently occluded (n = 5) or reperfused (n = 3) for 2 h after 2 h of LAD occlusion. In the permanent occlusion group, cellular debris was injected into the infarct center; the reperfused infarct group received cellular debris injections within the infarct or normal border zone of the myocardium. From these experiments, the late 111In kinetics from cellular debris is herein denoted as the debris impulse response function (DIRF).
Because cardiomyocytes have little turnover in normal tissue (17–20), canine myocardium was labeled in situ with 111In-tropolone to determine clearance characteristics of 111In from viable cells that did not die in vivo. Therefore, we hypothesized that labeling canine cardiomyocytes with 111In-tropolone would provide an indication of radiolabel loss from cells that are not dying at an appreciable rate in normal myocardium. After thoracotomy, 4 dogs were injected with free 111In-tropolone (mean ± SEM, 52.8 ± 17.1 MBq; 5–6 injections; 1 mL total volume) directly into the anterior surface of normal myocardium.
Finally, to obtain the total measured signal, viable BMSCs were injected in the peri-infarct region of reperfused infarcted myocardium. At the time of surgery, 3.08 × 107 ± 5.41 × 106 autologous canine BMSCs (3.69 ± 0.62 MBq; 1-mL volume; 5–7 injections) were intramyocardially injected (n = 4) after a 3-h LAD occlusion and reperfusion.
In Vitro Retention of 111In in BMSCs
In 2 separate experiments, canine BMSCs (1.76 × 106) were radiolabeled with 111In-tropolone, promptly plated, and incubated for 24 h to allow adhesion to culture plates. Supernatant activity containing 111In released from viable cells was measured daily using a high-purity germanium well counter (GWL; Ortec), and cell activity was measured using a γ-camera (Millenium MG; GE Healthcare). In both experiments, static images of cells were collected consecutively for several days after radiolabeling. Before each imaging session, cells were rinsed twice with 5 mL of PBS and replenished with fresh medium. Old medium and rinses were collected and measured using the well counter. Cell counts were corrected for physical decay and background and were fit to a monoexponential curve to determine the 111In biologic half-life for the imaging experiment. The cell viability after labeling and after the end of the experiments was assessed using trypan blue exclusion assay.
In Vitro Assessment of Nonspecific 111In Uptake
Nonspecific 111In uptake was assessed using the rat embryonic cardiomyoblast H9c2 cell line (CRL-1446; American Type Culture Collection), which served as an in vitro model of normal myocardium. To determine the uptake of 111In released after cell lysis, 1.3 × 106 BMSCs were labeled and lysed as described above. Radiolabeled lysates were then incubated with approximately 1 million H9c2 cells for 30 min, after which incubation activity was removed and cells were rinsed twice with 5 mL of PBS. Control H9c2 cells were labeled with 111In-tropolone as described. Cells were then trypsinized and collected for measurement using the well counter. Rinses and incubation activity were also measured.
The uptake of 111In lost from viable BMSCs by H9c2 cells was also evaluated. To do this, supernatant containing leaked 111In activity was collected from 111In-labeled BMSCs and incubated with H9c2 cells for 30 min. After incubation, the supernatant was removed and cells were rinsed with PBS and trypsinized. Counts from cells, supernatant, and rinses were measured using the well counter. Control H9c2 cells were labeled with 111In-tropolone.
SPECT Image Acquisition and Image Analysis
Serial SPECT and whole-body images were alternately acquired on anesthetized dogs using a dual-head Millennium MG equipped with medium-energy parallel-hole collimators. SPECT parameters were a 128 × 128 pixel matrix (4.52 mm2/pixel), 64 projections per head acquired over 180° (total 128 projections over 360°), and 171 and 245 keV (±10%) 111In energy windows. Whole-body imaging parameters were a 256 × 1,024 pixel matrix (with 2.26 mm2/pixel), a fixed acquisition time of 23 min, and the same 111In energy windows that were used for SPECT. SPECT and whole-body images of cellular debris and free 111In-tropolone injections were imaged serially on the day of injection; additional follow-up imaging was done for the free 111In-tropolone and viable cell injections over 3 and 2 wks, respectively. Subsequent follow-ups for these experiments required progressively longer SPECT image acquisitions of 30, 60, and 180 s/projection to obtain adequate count statistics in response to the decrease in the 111In signal.
SPECT projections were corrected for background and reconstructed using an iterative algorithm (21). VOI analysis was conducted on reconstructed SPECT images to generate time–activity curves, which were corrected for physical decay. In this analysis, the first SPECT dataset acquired (t = 0) had a VOI defined as pixels greater than or equal to 30% of the maximum pixel intensity and was used to create a mask image. This mask was then multiplied by each SPECT image acquired, and the mean pixel intensity was determined (MATLAB; MathWorks). Time–activity curves were fit to a biexponential function using MATLAB, and the short ( ) and long (
) and long ( ) components of the clearance curves were determined. Experiments involving viable BMSC transplantations were analyzed such that the last 3 data points were fit to a monoexponential function, and the resulting half-life was reported as the
) components of the clearance curves were determined. Experiments involving viable BMSC transplantations were analyzed such that the last 3 data points were fit to a monoexponential function, and the resulting half-life was reported as the  .
.
Modeling Transplanted Cell Viability Kinetics
To quantify the kinetics of TC survival within a VOI, we begin by stating that at any time, t, the total radiolabel signal measured (RLm(t)) by SPECT within the VOI comprises radiolabel released from dead TCs that remain in the VOI, RLd(t); radiolabel released from live TCs that remain in the VOI, RLl(t); and radiolabel within the surviving fraction of viable TCs, SF(t) (Fig. 1). We express each of these quantities in terms of the initial number of TCs (NTC) and the amount of radiolabel per TC at time 0, RLPTC. Additionally, RLm(t) is normalized to the sensitivity of the imaging system per TC and corrected for the radioactive decay of the radiolabel. Thus: Eq. 1Equivalently:
Eq. 1Equivalently: Eq. 2where each term ranges between 0 and 1. Our objective is to determine the shape of SF(t), which cannot be measured directly.
Eq. 2where each term ranges between 0 and 1. Our objective is to determine the shape of SF(t), which cannot be measured directly.
SPECT VOI (RLm(t)) containing radiolabel released from dead cells (RLd(t)), leaked from viable cells (RLl(t)) (C is fractional loss from viable cells), and in viable cells (SF(t)). DIRF describes kinetics of 111In after cell death.
First, we assume a functional form for the surviving fraction of TC, SF(t): Eq. 3where the half-life, TSF, is what we ultimately seek. We similarly assume a functional form for RLm(t):
Eq. 3where the half-life, TSF, is what we ultimately seek. We similarly assume a functional form for RLm(t): Eq. 4where Tm is the biologic half-life of the SPECT time–activity curve.
Eq. 4where Tm is the biologic half-life of the SPECT time–activity curve.
The term RLd(t) in Equation 2 considers radioactive label released from dead TCs and the removal of that label from the VOI. To account for this effect, we consider the number of TCs that have died from time 0 to time t, radioactive label—or debris—released from each dead cell, and kinetics of the debris retention at, and clearance from, the VOI. More formally: Eq. 5where SF(τ − dt) − SF(τ) describes the rate of change of SF(t) around time τ, DIRF(τ) is the DIRF at time τ, and ⊗ represents the convolution operator. Here, we assume that all radioactive label is released instantaneously on cell death—hence becoming debris—and immediately begins to clear from the VOI according to the kinetics of DIRF.
Eq. 5where SF(τ − dt) − SF(τ) describes the rate of change of SF(t) around time τ, DIRF(τ) is the DIRF at time τ, and ⊗ represents the convolution operator. Here, we assume that all radioactive label is released instantaneously on cell death—hence becoming debris—and immediately begins to clear from the VOI according to the kinetics of DIRF.
The final term in Equation 2, RLl(t), describes radiolabel leakage from surviving TCs, where leakage refers to the removal of 111In by any number of mechanisms, followed by subsequent clearance from the VOI according to DIRF. Thus: Eq. 6where the constant C describes the proportion of radiolabel inside a TC that leaves the TC and then begins to be cleared. Further, although the clearance of radiolabeled debris from dead cells may differ from radiolabel leaked from surviving cells, we have made the assumption that the clearance mechanism will be similar within the same tissue.
Eq. 6where the constant C describes the proportion of radiolabel inside a TC that leaves the TC and then begins to be cleared. Further, although the clearance of radiolabeled debris from dead cells may differ from radiolabel leaked from surviving cells, we have made the assumption that the clearance mechanism will be similar within the same tissue.
With RLd(t) and RLl(t) determined, we can correct the measured RLm(t) for the confounding effects of radiolabel from dead TCs and radiolabel leakage from the surviving fraction of TCs, thereby isolating the TC survival curve. More specifically, we determined the surviving fraction half-life, TSF, using a least-squares fit:
 Eq. 7
where the time integral is taken over the SPECT experimental time. Equation 7 was solved using the Marquardt–Levenberg iterative search algorithm, as implemented in MATLAB.
Eq. 7
where the time integral is taken over the SPECT experimental time. Equation 7 was solved using the Marquardt–Levenberg iterative search algorithm, as implemented in MATLAB.
We constructed a plot of TSF versus Tm to better appreciate the significance of the correction obtained by Equation 7. Using this plot, we estimated the TC survival curve half-life based on the radiolabel time–activity curve measured by SPECT. The TSF versus Tm plot has a minimum bound on Tm, defined as the variability of the half-life of DIRF because no radiolabel can clear faster than extracellular radiolabeled debris, and a maximum bound on Tm, defined by the variability in the half-life of the clearance of radiolabel from viable cells.
Experiments to measure DIRF(t) were repeated multiple times. Rather than averaging multiple DIRF curves, each DIRF curve was input individually to Equation 7, thus providing a range of possible TSF values for a given Tm. For a particular Tm, only those DIRF curves clearing faster than that Tm were considered to produce corresponding TSF values, which were then averaged and their SD calculated.
Statistical Analysis
Statistical analysis was performed using SPSS 15.0 (SPSS Inc.). Nonparametric tests were used for in vivo data that were not normally distributed, with corrections made for multiple pairwise comparisons. For cellular debris data, differences between injected tissue type (nonreperfused, normal, and reperfused) were determined using 2-way ANOVA. A 2-tailed test of significance detected differences between groups (significance set at α = 0.05). In vitro studies were analyzed using unpaired t tests, and exponential fits to in vivo data were assessed with goodness-of-fit statistics in MATLAB. All values are expressed as mean ± SEM unless otherwise indicated.
RESULTS
In Vitro Retention of 111In in BMSCs
BMSC viability was 93% after radiolabeling and had a labeling efficiency of 92%. After incubation activity was removed and cells were washed, the cellular 111In activity was 0.105 Bq/cell. Imaging revealed that retained 111In activity had a 14.1-d half-life (Supplemental Fig. 1; supplemental materials are available online only at http://jnm.snmjournals.org), and the subsequent viability at day 6 was 75%. Initial losses of 111In from viable BMSCs between days 0 and 1 were 17.6%; the average loss was 4.6% per day thereafter. Two independent experiments confirmed these results.
In Vitro Assessment of Nonspecific Uptake of 111In
We determined whether activity from lysed cells labeled surrounding cells with intact cell membranes in vitro. The inability of intact viable cells to take up 111In released from lysed cells after a 30-min incubation period was demonstrated. Figure 2A shows uptake of 111In from cellular debris in H9c2 cells, which had a labeling efficiency of 0.6% after the removal of 111In-labeled BMSC debris (52.2 ± 2.4 counts per second [cps] vs. 8,357.4 ± 837.1 cps; P < 0.01). The control cell group labeled with 111In-tropolone alone had a labeling efficiency of 68%. Similarly, 111In released by viable BMSCs and incubated with H9c2 cells demonstrated significantly less 111In associated with H9c2 cells, compared with the supernatant (0.79 ± 0.14 cps vs. 40.38 ± 0.86 cps; P < 0.001), as shown in Figure 2B.
Nonspecific uptake of 111In in H9c2 cardiomyoblasts. (A) After incubation of 111In-labeled BMSC cellular debris with H9c2 cells, compared with control H9c2 cells labeled with 111In-tropolone, no significant uptake of 111In in H9c2 cells was found (P < 0.001). (B) Evaluation of nonspecific uptake of 111In leaked from viable BMSCs indicated that significant amount of 111In remained within incubating supernatant, compared with H9c2 cells (P < 0.01).
In Vivo Acquisition of DIRF, RLl(t), and RLm(t)
Cellular debris experiments showed faster radiolabel clearance curves when injected under the same conditions as viable cells. Figure 3A shows time–activity curves from which  and
and  (i.e., DIRF) (Fig. 3B) for all cellular debris experiments were derived, giving values of 1.75 ± 0.36 h and 19.37 ± 4.05 h, respectively. When results were separated into infarcted and normal tissue injections, the
(i.e., DIRF) (Fig. 3B) for all cellular debris experiments were derived, giving values of 1.75 ± 0.36 h and 19.37 ± 4.05 h, respectively. When results were separated into infarcted and normal tissue injections, the  and
and  were not significantly different between these groups; this was also true for groups separated on the basis of cellular debris injected into reperfused or permanent infarctions. Figure 3C shows whole-body scans acquired at approximately 40, 240, and 340 min after the injection of 111In-labeled cellular debris into the infarcted myocardium of 1 dog, demonstrating 111In reduction in the heart and increased bladder activity over time.
were not significantly different between these groups; this was also true for groups separated on the basis of cellular debris injected into reperfused or permanent infarctions. Figure 3C shows whole-body scans acquired at approximately 40, 240, and 340 min after the injection of 111In-labeled cellular debris into the infarcted myocardium of 1 dog, demonstrating 111In reduction in the heart and increased bladder activity over time.
(A) Biexponential fits to time–activity curves (symbols represent normalized raw data) after injection of 111In-labeled cellular debris into canine myocardium (normal, reperfused, and nonreperfused; n = 11). (B) DIRF as calculated from long component of biexponential fits ( = 19.4 ± 4.1 h). (C) Whole-body scans from 1 dog injected with 111In-labeled cellular debris in peri-infarct region of infarcted myocardium. All images are scaled to maximal pixel count and qualitatively demonstrate biodistribution of labeled debris within injected heart (H), liver (L), kidneys (K), and bladder (Bl).
= 19.4 ± 4.1 h). (C) Whole-body scans from 1 dog injected with 111In-labeled cellular debris in peri-infarct region of infarcted myocardium. All images are scaled to maximal pixel count and qualitatively demonstrate biodistribution of labeled debris within injected heart (H), liver (L), kidneys (K), and bladder (Bl).
In experiments to determine 111In radiolabel leakage from cells that do not die, in situ labeling of host cardiomyocytes showed similar washout characteristics for  , whereas the
, whereas the  was longer than the
was longer than the  from the cellular debris injections. Figure 4A shows the time–activity curves used to derive
from the cellular debris injections. Figure 4A shows the time–activity curves used to derive  and
and  , which were calculated to be 1.34 ± 0.35 h and 882.66 ± 242.84 h, respectively. Figure 4B shows 111In biodistribution in whole-body scans from 1 dog after the injection of free 111In-tropolone in situ within normal myocardium on days 0, 7, and 15 and also demonstrates that most of the label remains within the injection site. Figure 4C is a SPECT/CT image showing the 111In label after injection into the anteroapical region of normal myocardium. Contrast-enhanced MRI of the excised heart (Supplemental Fig. 2), used to confirm the absence of myocardial damage due to these injections, demonstrated no uptake of gadolinium-diethylenetriaminepentaacetic acid and indicated no myocardial damage 21 d after the injection of 111In-tropolone in 2 dogs.
, which were calculated to be 1.34 ± 0.35 h and 882.66 ± 242.84 h, respectively. Figure 4B shows 111In biodistribution in whole-body scans from 1 dog after the injection of free 111In-tropolone in situ within normal myocardium on days 0, 7, and 15 and also demonstrates that most of the label remains within the injection site. Figure 4C is a SPECT/CT image showing the 111In label after injection into the anteroapical region of normal myocardium. Contrast-enhanced MRI of the excised heart (Supplemental Fig. 2), used to confirm the absence of myocardial damage due to these injections, demonstrated no uptake of gadolinium-diethylenetriaminepentaacetic acid and indicated no myocardial damage 21 d after the injection of 111In-tropolone in 2 dogs.
(A) Biexponential fits to SPECT time–activity curves after injection of 111In-tropolone into normal canine myocardium ( = 882.7 ± 242.8 h) (symbols represent normalized raw data). Dogs were serially imaged on injection day (see inset) and weekly thereafter. (B) Whole-body scans from 1 dog injected with 111In-tropolone showing radiolabel in heart on days 0 (d0), 7 (d7), and 15 (d15). All images are scaled to maximal pixel value. (C) SPECT/CT image of 111In radiolabel in normal myocardium of 1 dog localizing 111In injection within heart in transaxial and sagittal planes, respectively. L = liver; K = kidneys.
= 882.7 ± 242.8 h) (symbols represent normalized raw data). Dogs were serially imaged on injection day (see inset) and weekly thereafter. (B) Whole-body scans from 1 dog injected with 111In-tropolone showing radiolabel in heart on days 0 (d0), 7 (d7), and 15 (d15). All images are scaled to maximal pixel value. (C) SPECT/CT image of 111In radiolabel in normal myocardium of 1 dog localizing 111In injection within heart in transaxial and sagittal planes, respectively. L = liver; K = kidneys.
To determine RLm(t), canine BMSCs were injected into the myocardium and imaged with SPECT. The labeling efficiency was 73%, and average cellular activity was 0.12 ± 0.006 Bq/cell. Monoexponential fits to the data were good, and time–activity curves after viable BMSC transplantation in 4 dogs had an average  of 74.25 ± 7.55 h, as shown in Figure 5A. Images show whole-body scans of 1 dog injected with 111In-labeled BMSCs at days 3 and 7 after injection (Fig. 5B), with confirmation by SPECT/CT immediately after the injection (Fig. 5C) in an additional dog.
of 74.25 ± 7.55 h, as shown in Figure 5A. Images show whole-body scans of 1 dog injected with 111In-labeled BMSCs at days 3 and 7 after injection (Fig. 5B), with confirmation by SPECT/CT immediately after the injection (Fig. 5C) in an additional dog.
(A) SPECT time–activity curves showing long component of monoexponential fits for BMSC injections (Tl1/2 = 74.3 ± 7.6 h) (symbols represent normalized raw data). (B) Whole-body scans from 1 dog injected with 111In-labeled BMSCs within infarcted myocardium. Both images are scaled to maximal pixel count and show 111In activity remaining in heart (H) and liver (L) at days 3 (d3) and 7 (d7) after injection. (C) SPECT/CT images confirm presence of BMSCs in myocardium on transplantation day.
The measured biologic half-lives for all dogs in all groups are shown in Table 1.
Biologic Half-Lives from Canine Experiments
Modeling Transplanted Cell Survival
The cellular debris experiments facilitated the determination of the kinetics of 111In-labeled cellular debris from the SPECT VOI. The  was used in the model to obtain the kinetics.
was used in the model to obtain the kinetics.
In observing the true leakage fraction (C) from viable TCs, the effects of DIRF kinetics were removed. Although the rate of 111In clearance from the VOI was found to have an average leakage  of 36.8 d, we assumed this was not the true leakage because we modeled 111In outside viable TCs as clearing according to DIRF. Thus, the DIRF contribution was removed from the measured leakage clearance, and the adjusted leakage T1/2 was 38.5 d with C calculated to be 0.00075/h.
of 36.8 d, we assumed this was not the true leakage because we modeled 111In outside viable TCs as clearing according to DIRF. Thus, the DIRF contribution was removed from the measured leakage clearance, and the adjusted leakage T1/2 was 38.5 d with C calculated to be 0.00075/h.
The resulting Tm versus TSF curve corrected for cell death and leakage to determine TC survival is shown in Figure 6. At longer half-lives, RLl(t) affected the accuracy of the true cell activity because leaked activity accumulated outside viable cells but had not yet been removed according to DIRF. At short half-lives, the average DIRF clearance of 19.4 ± 4.1 h resulted in delayed cellular debris washout from the myocardium, thus affecting how well the model predicted the activity within intact cells. Therefore, the most accurate estimation of the true cell activity was between approximately 20 h and 37 d. The measured Tm from the viable cell transplantations was 74.3 ± 7.6 h and after corrections became 71.2 ± 6.4 h using this curve.
Plot of Tm vs. TSF shows calculated half-life of surviving fraction of TCs after corrections for radiolabel leakage (RLl(t)) and death (RLd(t)) kinetics are made to apparent half-life measured by SPECT (RLm(t)). Error bars represent SD, and ⋄ represents incorporation of another DIRF curve in estimation of TSF.
DISCUSSION
We have developed a quantitative SPECT method to image canine BMSC survival in vivo after radiolabeling the cells with 111In-tropolone and autologously transplanting them within the myocardium. In establishing this method, we had to determine the following: whether cellular 111In levels caused radiotoxicity, the retention of 111In in transplanted cells, the nonspecific uptake due to extracellular 111In activity, and the clearance kinetics of the interstitial radiolabel.
Our previous work showed that loading BMSCs by as much as 0.14 Bq/cell did not affect viability at 14 d and proliferation at 5 d (16). Work by others examining cellular 111In cytotoxicity (5,22,23) has suggested that greater amounts of 111In per cell can be used. Dosing studies suggest that loading cells with 0.18 Bq/cell (5) or 7.5 Bq/cell (23) does not compromise cellular function. Loading human mesenchymal stem cells with 7.5 Bq/cell did not affect proliferation capacity, endothelial cell differentiation ability, and surface protein expression patterns over 5–7 d (23). Thus, the 111In dose used here should produce minimal damage to canine BMSC function and viability. Although cellular fractionation studies have shown that 111In-oxine is confined mostly to neutrophil cytoplasm (80% of total cellular activity) (24), the range of Auger electrons that accompany 111In decay are in the nanometer to micrometer range (25). Thus, 111In likely has minimal effect on radiation-sensitive compartments such as nuclear DNA. Indeed, it has been shown that translocation of 111In, compared with cytosolic 111In, to the nucleus of breast cancer cells severely affects cell survival (26).
Several challenges remain in quantitatively imaging cellular viability using a nonspecific radiotracer. Radiolabel leakage from viable cells and nonspecific radiolabel uptake in the extravascular and extracellular space affect quantification. Tran et al. (27) demonstrated that 111In-oxine leakage from rat mesenchymal stem cells was initially high at 2 h after labeling but decreased by less than 1% per hour thereafter. 111In leukocyte labeling was also evaluated for label stability and had elution rates of 2% per hour (28). Our in vitro results indicated similar levels of stability in canine BMSCs, with 4.6% of activity lost per day. We also demonstrated 111In stability in viable cells in vivo. Because we were unable to control the viability of transplanted cells, we labeled endogenous cardiomyocytes in situ (because this population is largely postmitotic) (20). In these animals,  was approximately 883 h, and ex vivo gadolinium-diethylenetriaminepentaacetic acid MRI confirmed the absence of myocardial damage due to these injections, suggesting 111In labeling of intact viable myocardial cells. Cumulatively, these results suggest 111In-tropolone was retained within viable cells and subsequently provided an estimate of radiolabel leakage. Our leakage rate collectively represents all putative fractions of myocardial tissue due to radiolabel nonspecificity; however, average rates of 1.8% per day suggest 111In remains stable in viable cells. For quantitative imaging, we also needed to establish the extent to which 111In might be nonspecifically taken up by surrounding tissue as a result of cell death or radiolabel lost from viable cells. We addressed this by showing that the 111In from labeled stromal cellular debris was not significantly taken up by H9c2 cells in culture. Additional in vitro experiments also indicated that H9c2 cells incubated with leaked 111In from viable BMSCs was not taken up by H9c2 cells. These data suggest that quantitative viable cell imaging will not be affected by nonspecific radiolabel uptake in the surrounding myocardium.
was approximately 883 h, and ex vivo gadolinium-diethylenetriaminepentaacetic acid MRI confirmed the absence of myocardial damage due to these injections, suggesting 111In labeling of intact viable myocardial cells. Cumulatively, these results suggest 111In-tropolone was retained within viable cells and subsequently provided an estimate of radiolabel leakage. Our leakage rate collectively represents all putative fractions of myocardial tissue due to radiolabel nonspecificity; however, average rates of 1.8% per day suggest 111In remains stable in viable cells. For quantitative imaging, we also needed to establish the extent to which 111In might be nonspecifically taken up by surrounding tissue as a result of cell death or radiolabel lost from viable cells. We addressed this by showing that the 111In from labeled stromal cellular debris was not significantly taken up by H9c2 cells in culture. Additional in vitro experiments also indicated that H9c2 cells incubated with leaked 111In from viable BMSCs was not taken up by H9c2 cells. These data suggest that quantitative viable cell imaging will not be affected by nonspecific radiolabel uptake in the surrounding myocardium.
In vivo assessments of DIRF related to the time-dependent clearance of 111In-labeled dead cellular debris from myocardium was essential in developing a quantitative imaging model to assess cell viability. Our results indicated an average  of 19.4 h when labeled cellular debris was injected into normal and infarcted tissues. However, viable cells injected into infarcted myocardium cleared with a longer average
of 19.4 h when labeled cellular debris was injected into normal and infarcted tissues. However, viable cells injected into infarcted myocardium cleared with a longer average  of 74.3 h, suggesting the ability to distinguish between viable and dead cell injections with SPECT. As a result of the
of 74.3 h, suggesting the ability to distinguish between viable and dead cell injections with SPECT. As a result of the  of cellular debris, convolution-based modeling was used to remove its contribution to the overall 111In signal. Additionally, we tried to determine how myocardial blood flow might affect the DIRF by injecting labeled cellular debris into normal myocardium and infarcted myocardium that was either permanently occluded or reperfused but found no significant differences between these groups. Further investigation to accurately measure blood flow in more animals is warranted.
of cellular debris, convolution-based modeling was used to remove its contribution to the overall 111In signal. Additionally, we tried to determine how myocardial blood flow might affect the DIRF by injecting labeled cellular debris into normal myocardium and infarcted myocardium that was either permanently occluded or reperfused but found no significant differences between these groups. Further investigation to accurately measure blood flow in more animals is warranted.
Our time–activity curve analysis revealed 2 distinct phases occurring with 111In clearance from the VOI. Initially, we thought that the fast phase reflected viable cells dying at a faster rate. However, no significant differences with respect to this phase of myocardial radiotracer washout for all the experiments was observed. Our working hypothesis is that this initial rapid clearance phase did not reflect viable cells but rather radioactivity directly injected into a blood vessel or emerging from the needle track due to myocardial contraction. For this reason, only the long component was used in our model to quantify viable cell activity. However, the short component may provide important data on cell retention in the evaluation of different transplantation methods.
Extrapolating our work to the clinic would be feasible with SPECT images acquired within hours of transplantation, approximately 2–3 d later, approximately 3–4 d later, and the final images acquired at approximately 5–7 times the effective half-life determined from the second and third measurements. Provided that the initial imaging included a whole-body scan, this would suggest both the fraction of the transplanted cells remaining in the myocardium immediately after transplantation and their survival half-life.
This study has several limitations. In establishing the accuracy of modeling cell survival in vivo, clearance data derived from canine experiments identified an upper and a lower limit. At the lower boundary, because of the variability in the DIRF measurement the model cannot account for half-lives faster than dead cell clearance kinetics (DIRF). Similarly, at the upper end, half-lives that are longer than leakage estimates cannot be corrected using this model.
Although this model assumes no cell division within the transplanted cell population and we cannot reliably predict cellular proliferation, our method does not rely on the absolute quantification of cell number. Rather, it relies on the  , suggesting that the rate at which 111In signal is lost reflects changes in cell survival after corrections for death and leakage. Signal reductions would reflect the removal of 111In from the injection site, as shown by a shorter
, suggesting that the rate at which 111In signal is lost reflects changes in cell survival after corrections for death and leakage. Signal reductions would reflect the removal of 111In from the injection site, as shown by a shorter  ; the proliferation of healthy labeled cells, however, should result in the fractional sharing of the radiolabel without any loss, thus maintaining a constant signal.
; the proliferation of healthy labeled cells, however, should result in the fractional sharing of the radiolabel without any loss, thus maintaining a constant signal.
After infarction, macrophages accumulate within the first 24 h, with numbers peaking around 7 d (29). Such macrophages within infarcted tissue could phagocytose released label and remain within the injection site; hence, DIRF could vary with time after infarction. However, our current results suggest otherwise, because all 4 animals treated with cells demonstrated extremely good fits to the last 3 data points (r2 = 0.96–0.99). If DIRF changed dramatically with time after infarction, such excellent fits would be unlikely. Nevertheless, future work could evaluate macrophage effects on DIRF by injecting 111In-labeled cellular debris at later times after infarction.
In a novel approach to estimate radiolabel leakage in vivo from cells that do not die, we labeled normal cardiomyocytes in situ. This may not accurately reflect leakage from transplanted BMSCs, because leakage potentially differs between different cell populations. However, we decided that in vitro–acquired leakage estimates to correct in vivo data as previously done (27) could not adequately account for in vivo biologic complexity.
Ex vivo validation will be important to confirm the accuracy of our model.
CONCLUSION
This work suggests that 111In is a reliable marker of BMSC viability. We have shown in vitro that 111In is highly retained in this cell population and does not show nonspecific uptake in surrounding cells. SPECT indirectly confirmed these findings in canine myocardium, demonstrating that 111In-labeled cellular debris clearance is significantly faster than the clearance of 111In from in situ–labeled cardiomyocytes. Using these results, we have mathematically modeled the time-dependent cell survival, which may predict the benefits of cell therapies or evaluate strategies to improve transplant cell survival.
Acknowledgments
We thank Lela Deans, Dominique Ouimet, Jennifer Hadway, Eric Sabondjian, Yuan Jin, and Huafu Kong for technical assistance. We acknowledge funding from Canadian Institutes of Health Research (CIHR) (CIHR-MOP-9467), Ontario Research and Development Challenge Fund, Heart and Stroke Foundation of Ontario, and CIHR Vascular Training Program and an Ontario Graduate Scholarship in Science and Technology.
Footnotes
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication August 27, 2007.
- Accepted for publication February 12, 2009.













