Abstract
Radioimmunotherapy is an effective treatment for non-Hodgkin's lymphoma (NHL). 90Y-ibritumomab is an antibody targeting CD20 receptors on the surface of lymphocytes. We present observations from our clinical experience with 90Y-ibritumomab in the management of NHL. Methods: This was a retrospective study of 28 NHL patients treated with 90Y-ibritumomab. There were 21 men and 7 women, 36–85 y old. A diagnostic dose of 111In-ibritumomab was administered on day 0, and imaging followed immediately and at 24, 48, and 72 h. The doses of 90Y-ibritumomab ranged from 629 to 1,258 MBq (17–34 mCi). Outcomes were compared with the findings of the 111In-ibritumomab scans. Results: 90Y-ibritumomab induced objective responses in 22 of 28 patients. A complete response was noted in 9 patients, a partial response in 9 patients, and a mixed response in 4 patients. Three patients had stable disease, and 3 patients had disease progression. 111In-ibritumomab findings were positive in 19 patients and negative in 9 patients. A complete response was noted in 2 of 19 patients with positive findings and 7 of 9 with negative findings. A partial response was seen in 7 of 19 patients with positive findings and 1 of 9 with negative findings. Disease progression was observed in 3 of 19 patients with positive findings and 0 of 9 with negative findings. The remaining patients had a mixed response or no changes. Conclusion: A higher rate of complete response after 90Y-ibritumomab treatment was seen in patients with negative 111In-ibritumomab findings, whereas a higher rate of disease progression despite therapy was noted in patients with positive 111In-ibritumomab findings. This observation suggests that patients with bulky disease may require more aggressive management.
The American Cancer Society estimates that 66,120 new cases of non-Hodgkin's lymphoma (NHL) will be diagnosed in the United States in 2008. The estimated number of deaths from NHL is 19,160 for the same year (1). The classification of NHLs continues to evolve, and the World Health Organization incorporates, in its current classification, data derived from advances in the understanding of the pathogenesis of these disorders together with their distinguishing immunophenotypic, genotypic, clinical, and histopathologic characteristics (2). These advances allowed for significant improvements in survival of patients with NHL through complex combinations of chemotherapy, radiation therapy, and immunotherapy capable of reducing long-term toxicity without sacrificing efficacy.
Radioimmunotherapy has been shown in clinical trials to be an effective treatment for refractory or relapsed NHL. One of the available agents is 90Y-ibritumomab (Zevalin; Cell Therapeutics, Inc.), a monoclonal murine antibody targeting CD20 receptors on the surface of lymphocytes. We present observations from our clinical experience with 90Y-ibritumomab in the management of refractory or relapsed NHL.
MATERIALS AND METHODS
This was a retrospective study (January 2000–July 2006) of 28 patients with NHL who were treated with 90Y-ibritumomab for refractory or relapsed disease. The local Institutional Review Board approved this study. The group included 21 men and 7 women, with an age range of 36–85 y (average, 56.9 y). All patients were previously treated with a combination of rituximab (Rituxan; Biogen Idec Inc. and Genentech USA, Inc.) or R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone), external radiation therapy, or bone marrow transplantation and had stage III or IV disease.
A diagnostic dose (185 MBq [5 mCi]) of 111In-ibritumomab was administered on day 0 of the 90Y-ibritumomab therapy protocol. This dose was followed by pretherapy imaging consisting of whole-body planar anterior and posterior images acquired immediately and at 24, 48, and 72 h using an Infinia Hawkeye scanner (GE Healthcare) and interpreted using a dedicated Xeleris workstation provided by the manufacturer (GE Healthcare). The administered therapeutic doses of 90Y-ibritumomab (given on day 7 after the imaging dose) ranged from 629 to 1,258 MBq (17–34 mCi) (average, 1,054.5 ± 164.65 MBq [28.5 ± 4.45 mCi]). The patients were preloaded with unlabeled rituximab (250 mg/m2) on days 0 and 7. The protocol used for therapy is presented in Table 1.
Protocol for 90Y-Ibritumomab Therapy
The clinical outcomes of the 90Y-ibritumomab therapy were compared with the findings of the 111In-ibritumomab scans. Response to radioimmunotherapy was determined on the basis of all available clinical and radiographic follow-up, which included clinical notes, anatomic studies, and 18F FDG PET/CT scans. The response to therapy at 3 mo after 90Y-ibritumomab administration was assessed according to the EORTC criteria (3) for PET in 17 patients (60.7%). Determination of response was based on the referring physicians' overall impression from clinical notes. 111In-ibritumomab scans were recorded as positive if any known site of disease was identified and negative if no abnormal uptake was noted.
RESULTS
90Y-ibritumomab induced objective responses in 22 of the 28 patients (78.6%). A complete response was noted in 9 patients (32.1%), a partial response in 9 patients (32.1%), and a mixed response in 4 patients (14.3%). There were 3 patients (10.7%) with stable disease and 3 patients (10.7%) with disease progression. 111In-ibritumomab scans were available for review in all 28 patients: 19 had positive 111In-ibritumomab findings and 9 had negative 111In-ibritumomab findings. A complete response was noted in 2 of 19 patients with positive findings and 7 of 9 patients with negative findings. A partial response was seen in 7 of 19 patients with positive findings and 1 of 9 patients with negative findings. Disease progression was observed in 3 of 19 patients with positive findings and 0 of 9 with negative findings. The remaining patients had a mixed response or no changes. These findings are summarized in Table 2.
Correlation of 111In-Ibritumomab Pretherapy Scans and Outcomes of 90Y-Ibritumomab Therapy in Studied Population
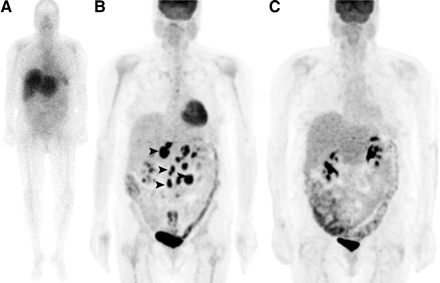
In Figure 1, we present a 60-y-old woman with NHL and a complete response after 90Y-ibritumomab treatment. No lesions were seen on the pretherapy 111In-ibritumomab scan (images from the 48-h scan are shown). Maximum-intensity-projection images of 18F-FDG PET (4 mo apart) showed resolution of the lesions noted before therapy. Figure 2 shows a 45-y-old man with NHL and progressive disease after 90Y-ibritumomab treatment. Lesions were seen in the abdominal region on the pretherapy 111In-ibritumomab scan (images from the 48-h scan are shown). Maximum-intensity-projection images of 18F-FDG PET scans (4 mo apart) show progression of the lesions noted before therapy.
A 60-y-old woman with NHL and complete response after 90Y-ibritumomab treatment. (A) No lesions are seen on anterior whole-body planar pretherapy 111In-ibritumomab scan (images from 48-h scan are shown). (B) Pretherapy (1 mo) MIP image of 18F FDG PET shows abdominal lesions (arrowheads). (C) MIP image of 18F FDG PET after therapy (3 mo) is negative for active disease.
A 45-y-old man with NHL and progressive disease after 90Y-ibritumomab treatment. (A) Lesions are seen in abdominal region (arrowheads) on anterior whole-body planar pretherapy 111In-ibritumomab scan (images from 48-h scan are shown). (B and C) Pretherapy (1 mo) MIP image of 18F FDG PET (B) and posttherapy (4 mo) MIP of 18F FDG PET (4 mo apart) (C) show progression of lesions noted before therapy (arrowheads).
DISCUSSION
The results of our retrospective review suggest that there may be an inverse relation between the amount of disease visible on the 111In-ibritumomab pretherapy scans and the response to 90Y-ibritumomab treatment, with a higher rate of complete response seen after 90Y-ibritumomab in patients with negative 111In-ibritumomab findings, and a higher rate of disease progression noted despite therapy in patients with positive 111In-ibritumomab findings. This observation contradicts prior reports that argue against giving the therapy for a tumor that has not been shown to take up the agent (4).
A limitation of our retrospective review is that 111In-ibritumomab is intended to assess biodistribution of the radiolabeled antibodies, not to be a sensitive method of disease detection. Thus, these observations need to be confirmed in a trial including proper imaging of the target CD20 receptors. One such attempt is a report by Perk et al., who labeled ibritumomab with 89Zr and reported the first use in a human subject. Their PET images obtained after injection of 89Zr-ibritumomab showed targeting of all known tumor lesions (5).
If we consider radioimmunotherapy as a step beyond immunotherapy of cancer, this step was prompted by the (relative) failure of the latter. The conventional way to explain the failure is a lack of intrinsic killing effect and a lack of penetration into poorly vascularized tumor masses. The addition of a radioactive label (usually a β-emitter) to the antibody would improve both. Radiation has the potential to cure, and the type of radiation used (β-rays) has a sufficient range to overcome the lack of penetration.
All available data, for all forms of disease, suggest that tumor doses are not high enough to have a curative effect and that measured tumor doses do not predict response (6). Yet there is, at least in lymphomas, a curative effect. This discrepancy has preoccupied researchers (7). Some of the magnified effect of what is effectively a relatively small radiation dose can be assigned to the continuous nature of the irradiation by radiolabeled antibodies (8,9). More important, whereas in external-beam irradiation an average dose to the tumor volume as a whole makes sense, the microscopic dose is heterogeneous (10). This heterogeneity may in fact increase the dose at a microscopic level to vital tumor elements. In addition, recent reports indicate a synergistic effect of rituximab followed by radioimmunotherapy, stressing the important role of unlabeled antibodies in the therapy of NHL (11).
Low-grade lymphomas are refractory to most treatments, and each subsequent treatment is less effective (12). Radioimmunotherapy seemed a possibility to improve the treatment options. At present, the most successful (and Food and Drug Administration–approved) radioimmunotherapy agents for lymphomas are anti-CD20 monoclonal antibodies. Rituximab is a chimeric antibody, used as a nonradioactive antibody and to preload the patient when 90Y-ibritumomab is used. 90Y-ibritumomab is the 90Y- or 111In-labeled form of ibritumomab tiuxetan. Bexxar (GlaxoSmithKline) is the 131I-labeled form of tositumomab. Ibritumomab tiuxetan and tositumomab are mouse anti-CD20 monoclonal antibodies. In neither case is the dose to the tumor calculated, because current in vivo imaging methods allow the calculation of only a macroscopic and average tumor dose, probably irrelevant in view of the microscopic heterogeneity. The administered quantity is limited by considerations of toxicity.
Horning et al. reported on a series of 40 patients treated with 131I-tositumomab (13). The median number of prior treatments was 4. Most patients had low-grade lymphomas (70%). Some had transformed low-grade (25%), and a few had intermediate-grade. Thirty-five (88%) had disease refractory to rituximab. The overall response rate was 68%, with a median response duration of 16 mo (1+ to 38+ mo). A complete response was seen in 33% of the patients. The median duration was not reached. Similar results were reported by Kaminski et al. about a series of 60 patients (14). Again, the patients included in this group received a median of 4 prior treatments. The low, transformed low, and intermediate grades were distributed as 56%, 38%, and 2%, respectively. Overall response and complete response were 47% and 20%, respectively, with median durations of 12 and 47 mo, respectively. When the method was used as the first treatment, the complete response rate was higher than 72% and the 5-y survival higher than 55% (15). In the context of other treatments and outcomes for low-grade B-cell lymphomas, these results are remarkable. Toxicity is primarily hematologic but is rare and reversible with the actual clinical protocols. Interestingly, only a single paper reported on a prospective and randomized study of the additional effect of the radioactive label (16). In that study, with the same amount of antibody in identical regimens but with one arm adding radioactivity, the complete response rate was 33% in the radioactive group and 8% in the unlabeled group. The overall response rates were 55% and 19%, respectively. Improvement will come by making the delivery more efficient by labeling the antibody, already joined to the target (17).
Other groups evaluated 90Y-ibritumomab: Conti et al. reported a low rate (0.6%) of altered biodistribution before therapy but did not comment on any role of the diagnostic scan in predicting the response to therapy (18). Visualization of disease on pretherapy imaging was not analyzed by Jacene et al. in a comparison of 131I-tositumomab versus 90Y-ibritumomab in clinical practice (19). However, Gokhale et al. suggested that bulky sites of disease on pretherapy scans could be used to establish a statistical hierarchy of areas likely to show tumor progression and applied this pattern to direct the use of additional external-beam radiotherapy to augment treatment (20). This is similar to the findings of our study that suggest that patients with bulky disease (identified even on pretherapy 111In-ibritumomab scans) may require more aggressive management.
CONCLUSION
A higher rate of complete response after 90Y-ibritumomab treatment is seen in patients with negative 111In-ibritumomab pretherapy findings, whereas a higher rate of disease progression despite therapy is noted in patients with positive 111In-ibritumomab findings. This observation, suggesting that patients with bulky disease (identified even on pretherapy 111In-ibritumomab scans) may require more aggressive management, needs to be confirmed in larger prospective trials using proper imaging of the target CD20 receptors.
Footnotes
-
COPYRIGHT © 2008 by the Society of Nuclear Medicine, Inc.
References
- Received for publication March 25, 2008.
- Accepted for publication July 8, 2008.