Abstract
Angiography of patients with typical chest pain reveals normal epicardial coronary arteries in about 20%. Coronary flow reserve (CFR) determination is an elaborate, but helpful, task, as only the evidence of microvascular disease enables appropriate therapy. We prospectively evaluated the incidence of a dysfunctional microcirculation and searched for predictive parameters of a reduced CFR. Methods: In 79 consecutive patients (52 females, 27 males) with typical angina and a normal angiogram and 10 control subjects (6 females, 4 males), CFR was measured by 13N-ammonia rest/dipyridamole PET and correlated with clinical parameters individually and summarized as the number of risk factors (NRF) using an elaborated cardiac risk factor score. Results: Sixty-five percent of patients had a reduced CFR (CFR < 2.5). CFR correlated with NRF (r = 0.55, P < 0.001), systolic blood pressure (r = 0.46, P < 0.001), interventricular septal thickness (r = 0.33, P < 0.01), and age (r = 0.25, P = 0.02). Eighty-five percent of patients with a high risk factor score (NRF ≥ 5) had a reduced CFR. In contrast, 100% of our patients with a low risk factor score (NRF < 2) presented a normal CFR. In total, 55% of our patients could be allocated to either one of these groups. Conclusion: In about two thirds of patients, anginal pain can be explained by a reduced CFR. Risk factors have a cumulative negative effect on CFR. A clinical cardiac risk factor analysis enables estimation of individual probability of microvascular dysfunction in a significant proportion of these patients. However, CFR measurements are recommended for those with an intermediate NRF.
The constellation of typical angina and a positive stress test in the presence of angiographically normal coronary arteries is found in about 20% of patients undergoing cardiac catheterization (1–4). In about 50% of these patients anginal pain is attributed to changes in coronary microvasculature (5–7).
In addition, microvascular dysfunction is independently predictive for cardiovascular events (8,9). Therefore, knowledge of coronary flow reserve (CFR) allows risk assessment and, consequently, initiation and monitoring of appropriate therapy (10–12).
However, the remaining 50% of these patients have noncardiac causes of chest pain, such as gastrooesophageal reflux, hernia, or degeneration of the skeletal system. Differential diagnosis between cardiac and noncardiac origin often is difficult but necessary for initiation of the appropriate therapy. In fact, a substantial number of these patients continue having chest pain after angiography and will undergo repeated coronary catherizations thereafter (13).
A defective microvasculature can be diagnosed only by determination of CFR. However, CFR measurements are either invasive or time-consuming or require highly trained medical personnel and extensive laboratory equipment. Therefore, diagnosis of microvascular angina can be performed only in highly specialized centers, enabling adequate work-up in only a small proportion of these patients.
Because several cardiac risk factors such as hypertension, hypercholesterolemia, smoking, and menopause have been shown to be related to microvascular dysfunction, an algorithm using these parameters could serve as a predictive model, possibly obviating the need for CFR measurements (7,14–25).
We therefore determined CFR in a consecutive series of patients with typical chest pain and a normal angiogram and assessed the value of cardiac risk parameters for prediction of microvascular dysfunction.
MATERIALS AND METHODS
Study Population
Seventy-nine consecutive patients (52 females, 27 males; mean age ± SD, 58 ± 10 y) with typical angina (defined as nitrosensitive retrosternal or precordial pressure induced by physical or emotional stress), a normal coronary angiogram < 3 mo old, and a positive stress test were included in the study. Seventy-nine percent of patients had a positive exercise test (≥0.1-mV rectilinear or downsloping ST-segment depression 80 ms after the J point in at least 2 leads); 21% of patients had reversible defects in thallium dipyridamole scintigraphy. Three study groups were analyzed: patients with a normal CFR (group I, CFR ≥ 2.5), patients with a reduced CFR (group II, CFR < 2.5), and 10 control subjects with normal coronary arteries (6 females, 4 males; mean age ± SD, 53 ± 11 y), atypical chest pain, a normal physical examination, rest electrocardiogram, and exercise test, and a low likelihood of coronary disease.
For exclusion of myocardial or valvular heart disease, transthoracic echocardiography composed of all standard views was performed on all subjects. Left ventricular dimensions, function, and mass were investigated according to standard criteria. Septal thickness (interventricular septal thickness [IVS]) was measured to evaluate left ventricular hypertrophy (>12-mm thickness) as an indirect sign of arterial hypertension. Patients with hypertrophic cardiomyopathy as well as patients with valvular abnormalities such as mitral valve prolapse were excluded from the study.
Blood sampling with determination of various coronary risk factors (cholesterol [including high-density lipoprotein and low-density lipoprotein], triglycerides, blood glucose, C-reactive protein [CRP], fibrinogen) and a questionaire (including characterization of chest pain, smoking, menopause in women, and family history of coronary artery disease [CAD], body mass index [BMI]) were included. Patients with diabetes mellitus, who have a considerably higher risk for significant artherosclerotic CAD and who also have a higher prevalence of microvascular disease (26,27), and patients with other major diseases were excluded from the study.
To examine the relationship between coronary risk factors and coronary reactivity, each risk factor was related to CFR. Additionally, according to a simplified score, including the most important risk factors for CAD, individual risk factors were summarized for each subject as the number of risk factors (NRF) with the following cutoff points:
Age ≥ 60 y
Menopause
Cholesterol ≥ 200 mg/dL
Triglycerides ≥ 170 mg/dL
Blood pressure at resting conditions: systolic > 140 mm Hg or diastolic > 90 mm Hg
IVS ≥ 12 mm
Smoking
BMI ≥ 25
Positive family history of CAD
Informed consent was obtained from all patients. All procedures were performed according to institutional guidelines and conformed with the principles outlined in the Declaration of Helsinki. The study protocol was approved by the local human subjects committee.
PET
All subjects were instructed to discontinue vasoactive medication and to abstain from caffeinated beverages and cigarettes for at least 24 h before undergoing the PET studies. Measurement of myocardial blood flow (MBF) was performed under resting conditions and under hyperemia using dipyridamole.
PET studies were performed on a dedicated PET system (Advance; GE Healthcare) starting with a 1-min scout scan for confirmation of correct patient's positioning and a 10-min transmission scan for attenuation correction. Then, 700 MBq 13N-ammonia were injected intravenously as a bolus. Simultanously, a 20-min, 18-frame, dynamic PET acquisition was started (12 frames × 10 s, 3 frames × 60 s, 3 frames × 300 s). Forty-five minutes after the first 13N-ammonia bolus, awaiting the physical decay of the 13N-ammonia activity, dipyridamole was infused intravenously at a rate of 0.56 mg/kg over 4 min. The second dose of 700 MBq 13N-ammonia was given 4 min after terminating the dipyridamole infusion when an adequate increase of rate·pressure product (RPP) had been achieved. Dynamic PET acquisition was again started as described.
Image Analysis and Calculation of MBF
Reconstruction of transaxial PET data was performed with a Shepp Logan filter, 10-mm full width at half maximum. Images were reoriented along the long axis of the heart using a commercial image-processing tool from GE Healthcare to obtain short-axis planes of the left ventricle (12 slices with a slice thickness of 4.25 mm). Further analysis of reoriented short-axis images was performed on an image analysis workstation (Onxy; Silicon Graphics Inc.) with an Interactive Data Language software package developed by Muzik et al. (28).
To generate time–activity curves for the blood pool and myocardium, regions of interest were drawn in 2 (for blood pool) and 4 (for myocardial activity) consecutive midventricular short-axis image planes. For the generation of myocardial time–activity curves, 12 sectorial regions were placed automatically at the endocardial border of the short-axis image planes (29). Dynamic datasets were analyzed using radial activity profiles. Time–activity curves were then fitted to get estimated values for MBF with a 3-compartment model developed by Hutchins et al. (30,31).
All time–activity curves were displayed in a polar map. After averaging all values of selected polar map elements, a single parameter was obtained characterizing averaged MBF.
CFR was defined as the ratio of hyperemic MBF (after dipyridamole) to resting MBF. Microvascular dysfunction was determined by a CFR below 2.5 (5,6). Additionally, CFR was corrected for RPP and expressed as normalized CFR (CFRn = [CFR × RPP]: 10,000) (32).
Statistical Analysis
ANOVA with the subsequent Fisher protected least significant difference test for multiple comparisons was performed using StatView 5.0.1 (SAS Institute) for comparison of data of various patient groups. To detect relationships between various risk factors and CFR, simple regression analysis was applied. Stepwise regression analysis was used to find independent parameters, building a model for prediction of CFR. All data are expressed as mean ± SD. Statistical significance was considered when P was < 0.05. Receiver-operating-curve (ROC) analysis was performed to differentiate between a normal and a reduced CFR in the dependence of NRF.
RESULTS
Clinical Characteristics
Clinical characteristics are depicted in Table 1. Although sex, serum lipids, BMI, and smoking habits did not significantly differ in the 3 study groups, patients of group II tended to be older and had a thicker interventricular myocardial septum than patients of group I and control subjects. Whereas women were predominant in all 3 groups, 50% of women in the control group, 78% of women in group I, and 85% of women in group II were postmenopausal. Familiy history of CAD was not significantly different in the 3 study groups. The number of cardiac risk factors was higher in group II than in group I and in control subjects. Forthy percent of control subjects, 67% of patients of group I, and 61% patients of group II were on a lipid-lowering therapy with statins.
Clinical Parameters, MBF, and CFR of Control Subjects, Patients with Normal CFR (Group I), and Patients with Reduced CFR (Group II)
Hemodynamic Parameters and CFR
Systolic blood pressure was significantly higher in group II compared with that in control subjects and in group I but was not significantly different between group I and control subjects (Table 1). Overall, 79 patients had a CFR of 2.37 ± 1.0; 28 patients (35%) had a normal CFR (group I, CFR = 3.46 ± 0.73) and 51 patients (65%) had a reduced CFR (group II, CFR = 1.77 ± 0.42). The control group (n = 10) had a CFR of 4.43 ± 0.77 (P = not significant vs. group I) (Table 1).
Correlations Between Clinical Parameters and CFR
Correlations between CFR, CFRn, and MBF under resting conditions and under stress and various clinical parameters are depicted in Table 2. NRF (r = 0.55, P < 0.001), systolic blood pressure (r = 0.46, P < 0.001), IVS (r = 0.33, P < 0.01), and age (r = 0.25, P < 0.02) correlated with CFR; none of the investigated laboratory parameters showed a significant correlation with CFR or MBF. The CFR of all patients (Fig. 1) and the number of patients with a reduced CFR (Fig. 2) were related to the NRF. In total, 85% of patients with a high risk factor score (NRF ≥ 5) had a reduced CFR.
(A) CFR of all patients vs. NRF. (B) Pearson correlation between CFR of all patients and NRF.
Relationship between number of patients with CFR < 2.5 and NRF.
Correlation Between Clinical Parameters and CFR, MBF at Rest, and MBF at Stress (n = 89)
When applying all of these parameters on stepwise regression, NRF had an R value of 0.42 (R2 = 0.18), obtaining the following model:
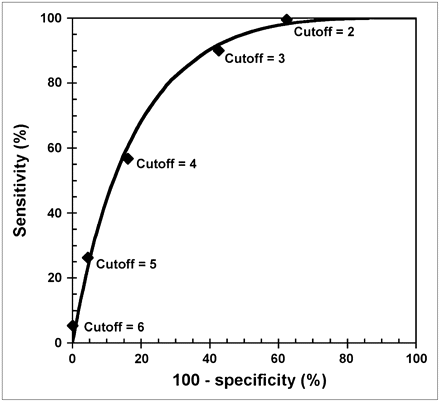
ROC analysis of NRF to differentiate between a normal and reduced CFR showed an area under the curve of 0.83 (Fig. 3). For prediction of a normal CFR, a cutoff point of 2 risk factors achieved a sensitivity of 100% and a specificity of 38%; a NRF of 6 was associated with a sensitivity of 5% and a specificity of 100%.
ROC analysis for NRF to differentiate between a normal and a reduced CFR.
DISCUSSION
The present study demonstrates that in patients with typical chest pain and a normal angiogram, a NRF < 2 or ≥ 5 enables prediction of microvascular disease with high accuracy. Therefore, a risk factor profile as used in the present study can obviate the need for costly and time-consuming CFR measurements in most of these patients. However, for the remaining patients with a NRF between 3 and 4 according to the used score, CFR measurements are necessary to evaluate microvacular function. Applying a statistical model including NRF enables an estimation of CFR.
In patients with typical angina, normal epicardial coronary arteries are found in about 20%. To differentiate cardiac from noncardiac causes of chest pain, evaluation of CFR is helpful for the patients' clinical management. However, in daily practice, the diagnosis “microvascular angina” often is an exclusion diagnosis and further investigations relating to the microcirculation are not implemented in the armamentarium of the average primary or secondary care hospital. CFR measurements are routinely performed only in highly specialized centers, allowing adequate workup in only a small proportion of these patients. As an alternative, a simple predictive model would be desirable to obviate the need for CFR measurement in daily routine. Because a series of established cardiac risk factors are known to correlate with CFR, we tested their value to predict microvascular dysfunction.
Risk Factors
Whereas isolated risk factors only weakly correlated with CFR, the number of CAD risk factors was closely related to CFR in the present study, demonstrating a cumulative negative effect of risk factors on CFR. Indeed, established CAD risk factors known to negatively influence coronary microvasculature were not significantly related to an impaired CFR when separately investigated. Hyperlipidemia (17,21,22,33) showed no significant relation to CFR in the present study, though it is known to influence microvasculature. Homogeneity of lipid levels probably caused by pretreatment with statins in a significant number of patients (40% of control subjects; 67% of patients of group I, and 61% of patients of group II were on statins) may explain the lack of correlation with CFR, particularly because of the positive effect of statins on microvasculature (34,35). Smoking (20) and menopause (7,25) showed a tendency, and age (36) was weakly related to CFR in our study, whereas the association of these factors has been shown previously. It must be emphasized that, in contrast to previous studies in which patients were specifically selected for having individual risk factors, the present study included a wide spectrum of consecutive patients as they are seen clinically without artificial or arbitrary preselecting criteria.
However, systolic blood pressure and myocardial wall thickness showed a clear relationship to CFR. Previous studies demonstrated the association of microvascular dysfunction and hypertension (18), especially in the presence of left ventricular hypertrophy (16,37). In fact, hypertension is associated with a higher resting MBF due to increased metabolic oxygen demand and reduced vasodilator capacity. Therefore, both pathologic resting and hyperemic blood flow are responsible for a reduced CFR in hypertensive patients (15). Whereas wall thickness reflects the degree of hypertension, systolic blood pressure shows the effectiveness of therapy. Consequently, when performing an individual risk factor profile for CFR estimation, wall thickness as well as systolic blood pressure should be taken into consideration for evaluation of the severity of arterial hypertension.
Prediction of Microvascular Disease Using a Risk Factor Profile
Amplification of CFR impairment when hyperlipidemia and hypertension coexist (14) led us to perform a score of conventional risk factors for CAD. The score established in the present study is arbitrary but summarizes most important conventional risk factors, is easy to determine, and gives an estimate of probability of microvascular dysfunction as a cause of chest pain.
In fact, in the present study the sum of risk factors for CAD is more closely associated with CFR as individual risk factors. Our score predicts microvascular dysfunction with high accuracy in 2 subgroups of patients: 85% of patients with a high-risk-factor score (NRF ≥ 5) had a reduced CFR. In contrast, 100% of our patients with a low-risk-factor score (NRF < 2) had a normal CFR. In total, 55% of our patients could be allocated to either one of these groups. Therefore, CFR measurements are necessary in only about 45% of patients presenting a NRF of 3–4 in whom microvascular function cannot be predicted accurately. Application of a statistical model including NRF allows an estimation of CFR. However, the impact of this model is reduced to the constellation of a clear trend of risk factor profile; otherwise, the estimated CFR value is in a grey zone of possible microvascular disease.
In particular, there exists a considerable variability in the magnitude of CFR in patients with 3 or 4 risk factors. This fact leads to the assumption that microvascular disease might be due to a cumulative effect of coronary risk factors, unknown variables, and genetic predispositions (9). This emphasizes the need for assessment of CFR by means of PET in these patients for proper risk stratification and therapy planning. Moreover, CFR measurements by PET would be a prerequisite for effective therapy monitoring in patients with known microvascular disease.
Comparison with Previous Studies
Previous studies investigating patients with chest pain and normal epicardial coronary arteries found a lower incidence of microvascular dysfunction ranging from about 30% to 50% in their patient population, whereas in the present study the proportion of patients with reduced CFR (not corrected for RPP) was 65% (5–7). This higher number of patients with microvascular disease is attributed to the fact that, in contrast to other studies, we did not investigate a preselected patient population; rather, we concentrated on the everyday patient with the constellation of angina and a normal coronary angiogram. In fact, our study population consisted of consecutive patients, and the prevalence of various cardiovascular risk factors was not an exclusion criterion from this study.
In contrast to the present study, our previously published study concentrated on the amount of blood flow abnormalities under resting and under hyperemic conditions in a smaller, but similar, study population. In 72% of patients with typical angina and normal epicardial coronary arteries, we found a reduced CFR when corrected for the RPP. Both abnormalities in resting and hyperemic MBF accounted for the reduction of CFR. Additionally, when investigated individually, coronary risk factors were not significantly different in control subjects, patients with normal CFR, and patients with reduced CFR (32).
In the Women's Ischemia Syndrome Evaluation (WISE) study, investigating only women with chest pain and no obstructive CAD, microvascular dysfunction was found in 47% of patients. Similar to our previously published study, there was no significant difference in indiviual risk factors or hormone levels, except age and the number of years after menopause, when comparing patients with normal and reduced CFR (7).
Limitations
The present study does not provide direct evidence that abnormal CFR is the cause of chest pain. Probably, a reduced CFR does not always account for clinical symptoms. Moreover, angina could also be related to excessive sensitivity to adenosine as a neural transmitter for cardiac pain, as about one third of the patients experienced discomfort during dipyridamole infusion. Additionally, the current results were collected from a group of patients referred for coronary angiography to evaluate persistent chest pain. Consequently, the prevalence of individuals with abnormal CFR but a normal coronary angiogram may be different than that among individuals being examined for chest pain in other settings. However, as demonstrated in our previously published study, most patients (72%) had a reduced CFR when corrected for the RPP (32). As all patients had significantly higher RPPs when compared with control subjects, a reduced CFR may correspond to a limited capacity for a further increase in CFR leading to angina.
CFR values were expressed as not corrected for the RPP, though it is known that CFR is strongly associated with cardiac work reflected by the RPP (36). On the other hand, increased cardiac work due to an inadequate RPP must also be seen as a pathologic entity itself, and CFR normalization to RPP would lead to artificial normal CFR values.
Estrogen status was not evaluated in the present study, as demonstrated in previous studies, suggesting a pathogenetic role (7,25). However, menopause was included and taken into consideration in the statistical evaluation.
CONCLUSION
A reduced CFR was found in 65% of consecutively investigated patients with typical angina and a normal coronary angiogram, proving the predominance of microvascular dysfunction in these patients. Risk factors have a cumulative negative effect on CFR. A clinical risk factor analysis enables estimation of individual probability of microvascular dysfunction in a significant proportion of these patients. However, CFR measurements are recommended for those with an intermediate NRF.
Acknowledgments
We are grateful for the support and technical assistance of the technicians in the PET Center of the Department of Nuclear Medicine at the Medical University of Vienna. We also thank Robert Dudczak, Department of Nuclear Medicine at the Medical University of Vienna, for his support and for his valuable comments on the manuscript.
Footnotes
-
COPYRIGHT © 2007 by the Society of Nuclear Medicine, Inc.
References
- Received for publication September 6, 2006.
- Accepted for publication November 2, 2006.