Abstract
Molecular imaging, in particular, PET, has brought an additional dimension to management for patients with cancer. 18F-FDG, which is the most widely available tracer, has been shown to be of value for the selection of target volumes in radiation oncology. Depending on its sensitivity and specificity, 18F-FDG has been shown to influence the selection of target volumes for non–small cell lung cancers (NSCLC) or for esophageal tumors. On the other hand, for tumors such as head and neck squamous cell carcinomas (HNSCC) and rectal carcinomas, convincing data on the value of 18F-FDG for target volume selection are still lacking. For target volume delineation, given that an adequate method is used for volume segmentation, the added value of 18F-FDG has been demonstrated for HNSCC and NSCLC. For both types of tumors, modifications in target volume delineation translated into differences in dose distribution compared with the results of CT scan–based plans. Studies are in progress for rectal tumors. Novel markers of tumor hypoxia or proliferation have the potential to modify the delineation of target volumes, allowing for “dose painting” in selected subvolumes. Finally, variations in tumor volume and viability during radiotherapy also are under intense investigation, potentially paving the way for so-called “theragnostic” or adaptive dose distribution during treatment. This review discusses how PET/CT might modify the current state of the art of radiotherapy planning.
Radiation oncology is fully integrated in the multidisciplinary treatment of cancer. It is estimated that 50% of all patients with cancer will benefit from radiotherapy during the course of their disease. Pivotal prospective or retrospective clinical studies have indeed demonstrated, with substantial evidence, the efficacy of radiotherapy as a sole treatment modality or in combination with other options, such as surgery, chemotherapy, and, more recently, biologically targeted agents (1). The improved efficacy of radiation treatment results in part from tremendous technological innovations that have been made available to the radiation oncology community over the last 50 y. It is reasonable to state that in 2006, 3-dimensional radiotherapy and intensity-modulated radiation therapy (IMRT) with on-board imaging capability have enabled the delivery of radiation doses with a degree of accuracy that has never been achieved before. These innovations have progressively contributed to the likelihood of improved local–regional control with reduced morbidity (2).
The implementation of 3-dimensional radiotherapy and IMRT requires knowledge of setup uncertainties, adequate selection and delineation of target volumes on the basis of anatomic or molecular imaging modalities, appropriate dose prescription and (dose) specification with regard to dose volume constraints, and quality control for both the clinical and the physical aspects of the entire procedure.
For target volume selection and delineation, anatomic imaging modalities, such as CT and, to a lesser extent, MRI, remain the most widely used modalities. CT is widely available, does not have geometric distortion, and provides intrinsic information on the electronic densities of various tissues—information that is used in dose calculation algorithms. As a limitation, CT lacks contrast resolution for normal soft-tissue structures and tumor extent. This limitation has led to significant inter- and intraobserver variations in delineation of the gross tumor volume (GTV) in head and neck, lung, esophageal, prostate, breast, cervical, and brain tumors (3–11). Furthermore, CT images are degraded by the presence of metallic structures, such as dental fillings; this property may significantly limit the performance of the modality in the assessment of oropharyngeal or oral cavity tumors.
MRI with various sequences (for example, unenhanced T1-weighted, contrast-enhanced T1-weighted, and T2-weighted sequences with or without the fat suppression option) is another anatomic imaging modality that can complement or sometimes replace CT. MRI has been shown to be more accurate than CT for evaluating the soft-tissue or bone extent of nasopharynx, prostate, and brain tumors (5,12,13). This advantage is translated into smaller interobserver variations in delineation of the GTV compared with the results obtained with CT (13–15). However, for pharyngeal–laryngeal tumors, the advantage of MRI over CT has not been confirmed, either in terms of interobserver variability or in terms of target volume delineation (16,17).
Over the last few years, the use of molecular imaging, particularly the use of positron-labeled 18F-FDG, has become increasingly popular in oncology. Given that adequate tracers are used, molecular imaging with PET enables visualization of the various molecular pathways of tumors, including metabolism, proliferation, oxygen delivery and consumption, and receptor or gene expression. Applied in the clinic, PET can be useful for tumor staging, for prediction of the tumor response, for selection or delineation of radiotherapy target volumes, for assessment of the tumor response to treatment, for the detection of early recurrence, or as a tool to evaluate modifications in organ function after treatment (18). The use of PET in general and of PET with 18F-FDG in particular for radiotherapy planning purposes has taken on increasing importance, so that more and more radiation oncologists believe that target volume selection and delineation cannot be adequately performed without the use of PET with 18F-FDG. Part of the attraction of PET can be attributed to the fact that the use of new tools is naturally fashionable and appealing. However, apart from this aspect, why should metabolic information be considered more important than the anatomic information provided by CT or MRI? What is the evidence supporting the use of 18F-FDG in the treatment planning process?
The objective of this article is to review the use of PET in radiotherapy planning, with special emphasis on its application for head and neck, lung, esophageal, and rectal tumors. The following aspects are discussed: specific issues related to the use of PET in radiotherapy planning; specific aspects of the use of 18F-FDG in radiotherapy planning for head and neck, lung, esophageal, and rectal tumors; and challenging issues related to the use of 18F-FDG and other tracers in radiotherapy.
SPECIFIC ISSUES RELATED TO USE OF PET IN RADIOTHERAPY PLANNING
The goal of the planning process is to select and delineate target volumes (and organs at risk) on the basis of all of the available diagnostic information and on the knowledge of the physiology of the disease, that is, the probability of local and nodal infiltration. This goal is achieved in part through the use of various imaging modalities, which depict more or less accurately the true tumor extent. The difficulty with imaging modalities is that none of them has a sensitivity of 100% (no false-negative examinations) or a specificity of 100% (no false-positive examinations). Thus, false-negative and false-positive results for depicting neoplastic processes occur.
How the sensitivity and specificity of a particular imaging modality influence the radiation planning process depends on the underlying objective of the treatment. If, for a particular disease, the objective is to avoid missing a tumor at any expense, a highly sensitive approach needs to be selected. Such a selection will, of course, result in a lower specificity and in the inclusion of nonneoplastic tissue in the target volume. However, this approach reduces the likelihood of missing neoplastic cells. If, on the other hand, the aim is to avoid including nonneoplastic cells in the target volume to protect normal tissue, a highly specific approach needs to be elected. However, such an approach reduces sensitivity and increases the risk for missing tumor cells.
When a novel imaging modality (for example, PET with the tracer 18F-FDG) is introduced, its sensitivity and specificity need to be compared with those of the standard test; for radiotherapy planning, the standard test is CT. Furthermore, its potential impact on treatment planning needs to be determined. For example, if an additional lymph node is visualized with a new imaging modality known to be more specific than the standard modality, it may be legitimate to increase the target volume(s) beyond what would have been used with a standard procedure; conversely, if fewer nodes are visualized with a new imaging modality known to be more sensitive than the standard modality, it may be legitimate to decrease the target volume(s) below what would have been used with a standard procedure. Table 1 summarizes the available data on the specificity and sensitivity of 18F-FDG PET and CT for the staging of lymph node involvement in lung cancer, head and neck cancer, cervical cancer, and esophageal cancer. For the analysis shown in Table 1, surgical lymph node sampling was used as the gold standard.
Comparison of CT and 18F-FDG PET for Staging of Lymph Node Involvement
For head and neck tumors, as shown in Table 1, CT and 18F-FDG PET performed with comparable diagnostic accuracies (19–25). A potentially interesting use of 18F-FDG PET is staging for patients with nodes found negative (node negative) by other imaging modalities, in whom the issue could be to avoid treating neck nodes if an 18F-FDG PET examination is negative. However, data have indicated that in the node-negative neck, the sensitivity of 18F-FDG PET, compared with that of examination of a pathologic specimen after neck node dissection, is only about 70% (24). These data are not surprising in light of the fact that in node-negative patients who underwent a prophylactic neck node dissection, microscopic nodal infiltration can be observed in up to 30% of cases (26). The rather low signal-to-background ratio of 18F-FDG and the limited spatial resolution of PET preclude the detection of microscopic disease by PET. In conclusion, compared with anatomic imaging modalities such as CT and MRI, 18F-FDG PET is not likely to be superior for the selection of the target volume in neck lymph nodes.
In contrast, when the added value of 18F-FDG PET for non–small cell lung cancer (NSCLC) is evaluated, the situation is the opposite. The sensitivity for the staging of lymph node involvement in lung cancer is significantly higher for 18F-FDG PET than for CT. These data imply that a negative PET examination could result in substantially reduced target volumes and permit focusing on the primary tumor (27–31).
For esophageal cancer, the sensitivity of 18F-FDG PET is similar to that of CT. Conversely, 18F-FDG PET is very specific for the staging of lymph node involvement outside the mediastinum, for example, supraclavicular or celiac lymph nodes (32). When such lymph nodes are detected by 18F-FDG PET, it is legitimate to increase the selection (and thus the delineation) of the target volume (33).
For paraaortic lymph nodes in patients with cervical carcinoma, 18F-FDG PET was also reported to be much more specific than CT or MRI, although this finding was based on a limited number of patients. These data support the inclusion of these nodes in cases of positive PET findings (34–36).
All of the data mentioned so far were obtained with PET cameras, whereas more and more centers are being equipped with dual PET/CT systems. Few systematic comparisons of the diagnostic accuracies of stand-alone PET and integrated PET/CT have been performed. Overall, diagnostic accuracy has been improved with the use of dual PET/CT cameras (37–43). However, it is interesting to note that, although logistically more demanding, PET and CT performed almost as well as dual systems in a side-by-side comparison (37). Whatever the improved diagnostic accuracy of dual PET/CT examinations, the results of more extensive comparisons between PET/CT and PET are needed before definitive conclusions can be drawn from the available data.
In conclusion, in contradiction to what has been reported by others (44), the use of 18F-FDG PET for the selection of target volumes should be accepted depending on the intrinsic diagnostic accuracy of this imaging modality for various disease sites and on the objectives of the treatment.
PET WITH 18F-FDG IN RADIOTHERAPY PLANNING FOR HEAD AND NECK SQUAMOUS CELL CARCINOMA (HNSCC)
As discussed earlier, the value of 18F-FDG PET for the selection of target volumes in the head and neck area is yet to be proven. Indeed, its sensitivity and specificity for the assessment of head and neck node infiltration do not differ significantly from those of CT (Table 1). However, a recent study of 20 patients with mostly locally advanced disease demonstrated an increase in sensitivity with hybrid PET/CT compared with CT alone (45). The authors showed that PET/CT-based radiation treatment would have significantly changed the dose distribution (46). These findings need to be confirmed prospectively with larger study populations before this technique can be implemented in routine use.
Although the use of PET for the delineation of the primary tumor is becoming increasingly popular, the accurate determination of the volume and shape of the tumor from PET images still remains a challenging task and an incompletely resolved issue. Although most of the reports regarding the segmentation of PET images have dealt with NSCLC, various methods already have been tested to determine the outline of 18F-FDG–positive tissue in patients with HNSSC. The easiest and simplest method consists of visual interpretation of PET images and definition of the tumor contours by an experienced nuclear medicine physician or a radiation oncologist. However, this method, which was applied in 2 recent studies, appears to be highly debatable (44,47). First, the threshold level of the PET image, which depends on the display windowing, strongly influences the visual assessment of tumor boundaries. Moreover, the visual delineation of objects is a subjective approach that necessarily leads to substantial intra- and interobserver variabilities.
In this framework, the development of objective and reproducible methods for segmenting PET images has become crucial. The simplest method relies on the choice of a fixed threshold of activity, that is, a given percentage of the maximal activity within the tumor, for distinguishing between malignant and surrounding normal tissues. Using a fixed threshold of 50% of the maximal activity to automatically segment primary tumors in the head and neck region, Paulino et al. showed that tumor volumes delineated from PET images obtained with 18F-FDG were larger than those delineated from CT images in 25% of the cases (48). Results from that study must be interpreted with caution because the relevance of an arbitrary fixed threshold appears to be highly questionable. Indeed, it has been shown that an adequate threshold for fitting macroscopic laryngectomy specimens, used as the gold standard, varied among specimens by between 36% and 73% of the maximal activity (49). Along with the absence of validation studies, these data clearly illustrate that fixed threshold–based methods are definitely not adequate for accurately segmenting head and neck tumors from PET images and should therefore be avoided.
The use of an adaptive threshold is an elegant option that could eliminate the drawbacks of methods that we have already described. We previously validated a threshold-based method with a dedicated phantom (50). The method relies on a model that determines the appropriate threshold of activity on the basis of the signal-to-background ratio. This method was shown to be accurate for segmenting PET images of a series of pharyngeal–laryngeal tumors (16). In that study, a quantitative comparison of CT, MRI, and PET with 18F-FDG showed that automatic segmentation of PET images led to tumor volumes that were significantly smaller than those obtained by either CT or MRI. Moreover, these 18F-FDG–determined volumes were by far the closest to the reference volume assessed from the surgical laryngectomy specimens. Although this method has been validated as a reliable segmentation method, it has some drawbacks. For instance, it is unclear whether this method is valid across different centers (51,52). Also, this method is not ideal for images with low signal-to-background ratios, such as those encountered with peritumoral inflammation induced by radiotherapy or with undifferentiated tumors.
The use of gradient-based segmentation is a method that was motivated mainly by the rather inaccurate definition of tumor edges by 18F-FDG PET. Gradient-based methods, which are used for CT, cannot be used for PET because of its poor resolution. Image restoration tools, such as edge-preserving filters and deconvolution algorithms, generate high-quality images that affirm the use of gradient-based segmentation techniques. Preliminary experiments showed that the segmentation of phantom objects and head and neck tumors on the basis of a combination of watershed transform and hierarchical cluster analyses provided encouraging results (53). The main advantage of such a method is that it is purely data driven; no underlying model or calibration curve is necessary. Consequently, both the applicability and the extent of use of such a method could be increased because it could still yield reasonable segmentation in difficult cases (e.g., images with low signal-to-background ratios) in which threshold-based methods usually fail. A typical example is the use of 18F-FDG PET during radiotherapy. The combination of the radiotherapy-induced mucositis, which increases the background signal, and the reduction in tumor uptake secondary to the treatment response leads to a drastic decrease in the signal-to-background ratio. In this context, the differentiation of the residual tumor from the surrounding inflammatory area requires either powerful segmentation methods, such as gradient detection or clustering analysis, or delayed image acquisition (54). In this framework, experimental data have suggested that the kinetics of 18F-FDG uptake differ between tumor and surrounding inflammatory cells (55). These differences might be exploited through dynamic PET acquisitions leading to different time–activity curves for inflammatory tissues and tumor. Although this concept appeared to be promising and has been successfully applied for the detection of early tumor recurrences, preliminary studies performed with animal models mimicking tissue inflammation and with patients treated with radiotherapy for head and neck cancer did not confirm its potential advantage (56; Xavier Geets, unpublished data, July 2005).
A challenging aspect of the use of 18F-FDG PET for HNSCC is the potential consequence on dose distribution of PET-based plans compared with CT-based plans as routinely performed. It was recently reported that PET-based plans led to a significant reduction in the volumes of the high doses, thus limiting the dose delivered to the surrounding normal tissues at risk (57). This observation could have important consequences, as it paves the way for a possible increase in the prescribed dose for the target volume.
PET WITH 18F-FDG IN RADIOTHERAPY PLANNING FOR NSCLC
18F-FDG PET stages local–regional and distant disease involvement in patients with lung cancer with a high degree of accuracy and affects management for approximately one third of patients (27,58,59). Furthermore, it results in a lower rate of futile thoracotomies. Finally, by detecting distant metastases, PET has the ability to exclude patients from radical therapy (60).
Here we examine the influence of PET on the selection and delineation of target volumes, address methodologic problems, and elucidate whether PET can help in better assessing the tumor response to radiotherapy.
18F-FDG PET has higher sensitivity and specificity for the staging of lymph node involvement than CT and may thus alter the GTV either by detecting unnoticed metastatic lymph nodes or by downstaging a CT–false-positive mediastinal lymph node. The second situation seems to be more frequent. For a series of 44 patients, De Ruysscher et al. showed that 18F-FDG PET altered the stage of the disease in 11 patients (25%) by downstaging the disease in 10 of these patients (61). As a consequence, the GTV based on PET with 18F-FDG was on average smaller than the GTV defined by CT. In a simulation study, the same group showed that for the same expected levels of toxicity to the lungs, spinal cord, and esophagus, the dose delivered to the tumor could be increased by 25%, resulting in a potentially higher tumor control probability (24% for PET/CT planning compared with 6.3% for CT-only planning) (62). In addition to better detection of true-positive lymph node involvement, 18F-FDG PET further alters the definition of the GTV by discriminating tumor tissue from atelectasis or necrosis (Fig. 1) (63,64). Other investigators reported that 18F-FDG PET alters the GTV in 22%–62% of patients (65).
Role of 18F-FDG PET in delineating volume of lung cancer. (A) Axial, coronal, and sagittal CT slices from patient with lung cancer located in left hilar region, associated with retroobstructive atelectasis of entire left lung, and associated with major pleural effusion. (B) Metabolic information provided by 18F-FDG PET shows that tumor tissue is confined to hilum. Delineation of tumor margin is easier and more accurate with help of 18F-FDG PET, allowing for significant modification of target volume.
To date, only a few studies have prospectively included PET with 18F-FDG in radiotherapy planning and addressed its impact on local control and survival, which is the ultimate question that one has to answer before incorporating molecular imaging into routine radiotherapy planning processes. Interestingly, selective mediastinal lymph node irradiation based on PET with 18F-FDG yielded a low rate of treatment failure for isolated nodes, suggesting that reducing the target volume does not result in poorer local control (61). In a modeling study, van Der Wel et al. reported that for 21 patients with N2 or N3 NSCLC, the use of PET/CT in radiotherapy planning resulted in a lower level of radiation exposure of the esophagus and the lungs, allowing a significant increase in the dose delivered to the tumor (66). Finally, PET, especially PET/CT, imaging has another positive effect on tumor volume delineation. Many groups noted that the interobserver variability, as well as the intraobserver variability, was significantly reduced when the 18F-FDG PET image was available for tumor volume delineation (67,68).
Some issues related to the use of PET for lung cancer radiotherapy are still unresolved. First, tumor volume delineation or contouring by PET is still unsatisfactory, as discussed earlier for head and neck cancers. The appropriate activity threshold to be used to automatically delineate the tumor contours varies with the size of the tumor and the tumor-to-background ratio (69). Recently the suggestion was made to include in the GTV defined from PET images the area of lower uptake (which was designated the “anatomic biologic halo”) immediately surrounding the most metabolically active part of the tumor. Including this halo resulted in better dose coverage of the planning treatment volume (PTV) (67). Standardization is needed because the use of different delineation techniques for 18F-FDG PET leads to different GTVs (51).
The second methodologic issue, of particular importance in lung cancer radiation therapy, is tumor motion during PET and radiotherapy. PET images are usually acquired during free breathing. The usual emission scan duration for conventional PET is 5–10 min per bed position. For PET/CT, a short CT scan is used for attenuation correction. To increase the accuracy of tumor volume delineation, respiratory gating techniques should be implemented (70).
PET WITH 18F-FDG IN RADIOTHERAPY PLANNING FOR ESOPHAGEAL TUMORS
18F-FDG PET is particularly specific for the detection of lymph node involvement outside the mediastinum, a feature that may help to optimize the radiation target volume (Fig. 2) (32). Vrieze et al. analyzed the additional value of PET with 18F-FDG for optimization of the clinical target volume (CTV) in 30 patients with advanced esophageal cancer (33). Discordances between conventional staging modalities, including CT and endoscopic ultrasound (EUS), for the detection of lymph node involvement were found in 14 of 30 patients (47%). In 8 patients, the involved lymph nodes were detected only by CT and EUS, and this finding would have led to a decrease in the CTV in 3 of them. On the other hand, PET with 18F-FDG was the only technique that detected lymph node involvement in 6 patients, resulting in a possible larger CTV in 3 of them (10%). The authors concluded that the high specificity of PET with 18F-FDG for the detection of lymph node involvement justifies its use for treatment volume adaptation in the presence of positive findings, whereas the low sensitivity of PET with 18F-FDG, that is, false-negative lymph node involvement, would result in an erroneous reduction in the CTV. Whether the role of PET with 18F-FDG in treatment planning will lead to a therapeutic gain without increasing toxicity remains unanswered.
PET/CT with 18F-FDG shows paratracheal adenopathy proven to be malignant by fine-needle aspiration cytology for patient with esophageal cancer.
Another study evaluated the impact of CT and 18F-FDG PET on conformal radiotherapy in 34 patients with esophageal carcinoma and referred for radical chemoradiation. After manual delineation of the GTV by both modalities, CT and PET images were coregistered. Image fusion (the PET-based GTV was used as an overlay for the CT-based GTV) resulted in a decrease in the GTV in 12 patients (25%) and an increase in 7 patients (21%). Modification of the GTV affected the PTV in 18 patients and affected the percentage of the lung volume receiving more than 20 Gy in 25 patients (74%), with a dose decrease in 12 patients and a dose increase in 13 patients (71). A similar study was performed with an integrated PET/CT scanner. Here, the GTV was enlarged in 9 of 10 patients (90%) by a median volume of 22% (range: 3%–100%) when information from PET with 18F-FDG was added to the CT-based GTV. In 3 patients, PET-avid disease was excluded from the PTV defined by CT; this finding would have resulted in a geographic miss (72).
The possible role of 18F-FDG PET/CT in radiotherapy planning for esophageal cancer was further confirmed by Howard et al. (73) for 16 patients. CT-derived GTVs were compared with GTVs contoured on PET/CT images by means of a conformality index. The mean conformality index was 0.46, suggesting a significant lack of overlap between the GTVs in a large proportion of patients. The use of PET/CT in treatment planning for patients with esophageal cancer is now being evaluated in a prospective trial (74). Preliminary findings on incorporating EUS and PET in the treatment planning process for 25 patients with esophageal carcinoma showed that the measured tumor length was significantly longer on CT than on PET with 18F-FDG (74). The authors concluded that PET could be of additional aid in treatment planning. Although EUS measurements of the tumor length were as accurate as PET measurements, the results of EUS are difficult to translate into the planning process. A major drawback of this study was the lack of comparison with pathologic findings after surgery. Pathologic examination is, however, not easy, because chemotherapy and radiation induce tissue changes, which can hamper the interpretation of images. Whether the role of PET with 18F-FDG in more accurate delineation of target volumes will lead to a therapeutic gain remains unanswered.
PET WITH 18F-FDG IN RADIOTHERAPY PLANNING FOR RECTAL CANCER
In many centers, preoperative chemoradiation is the standard treatment for locally advanced rectal cancer. The CTV includes the primary tumor, regional lymph nodes, and pelvic areas at risk for subclinical disease (75). Accurate dose delivery and the possibility of modulating the dose prescription with IMRT pave the way for the use of molecular imaging as a promising tool for selecting specific areas in a tumor that may be more radiation resistant (Fig. 3).
Imaging studies performed before and during course of treatment for patient with rectal cancer. (Upper row) Images from CT performed in prone position before chemoradiotherapy (RT), during chemoradiotherapy, and at time of surgery. (Middle row) Images from MRI performed in supine position before chemoradiotherapy, during chemoradiotherapy, and at time of surgery. (Lower row) Images from 18F-FDG PET performed before chemoradiotherapy, during chemoradiotherapy, and at time of surgery. Tumor (red arrows) shows high level of uptake of 18F-FDG before start of treatment; 18F-FDG signal decreases during treatment and is lowest at time of surgery. 18F-FDG PET can help in delineating GTV before treatment and in replanning radiation treatment during course of treatment. White arrows indicate urinary bladder.
The usefulness of PET with 18F-FDG has been investigated for the initial staging of colorectal cancer (76–78). All of those studies suggested that preoperative PET is useful for the diagnosis of the primary tumor but is of limited value for detecting metastasis to the regional lymph nodes. The potential use of PET/CT in radiotherapy planning for rectal cancer was addressed in 1 study (79). The authors evaluated the accuracy of target volume definition by PET with 18F-FDG for 11 patients by using an integrated PET/CT system. They found that the PET-defined GTV did not seem to correlate well with the pathologic tumor volume. However, this study must be interpreted with great caution, as several methodologic weaknesses were present. First, the authors compared pretreatment PET/CT images with pathologic specimens obtained after preoperative chemoradiotherapy. Second, they used a fixed threshold and a so-called standardized region-growing algorithm to segment the target volume. By doing so, they definitely omitted the selection and delineation of the CTV, which contains all pelvic regions that are at risk for subclinical disease in rectal cancer, including the internal iliac lymph node regions (75).
Even if PET can provide additional functional information, its usefulness in the treatment of rectal cancer is still questionable and needs to be evaluated in prospective trials with strict methodology. Its benefit may be of little interest in preoperative 3-dimensional conformal radiotherapy, as the total mesorectum included in the CTV will be surgically removed anyway. However, it may be more important when “dose painting” in relevant biologic regions needs to be achieved with simultaneous integrated boost techniques. Whether this benefit in turn can improve patient outcome must be proven in future trials. Moreover, problems that require specific attention remain; these include image coregistration and variations in patient setup, in organ motion, and in organ shape. Integrated PET/CT is the modality of choice for more accurate registration. However, small variations are still possible because of the elastic properties of the rectum wall, causing distortions of the rectum (and tumor) during the time of acquisition (Fig. 4). Displacements of the rectum wall or tumor could also induce geographic misses when the application of higher doses to small volumes is planned.
Correlation of resection specimen with different imaging modalities for patient with rectal cancer. (Column 1) Macroscopic section through rectal cancer resection specimen from top to bottom. (Columns 2–4) Correlating imaging studies: MRI, CT, and 18F-FDG PET, respectively (all performed in prone position). This figure illustrates how molecular imaging modalities can be validated by correlation with pathologic specimen.
FUTURE PROSPECTS: “THERAGNOSTIC” IMAGING FOR RADIATION ONCOLOGY
Recent developments in molecular imaging have created opportunities to unravel the complexity of tumor biology. In addition to 18F-FDG, which is likely a surrogate for tumor burden and hence for clonogenic density, tracers of hypoxia, such as fluoromisonidazole, 3,3,3-trifluoropropylamine, fluoro-erythronitroimidazole, or copper-diacetyl-bis(N4-methylthiosemicarbazone) (copper-ATSM); of proliferation, such as 5-bromo-2′-fluoro-2′-deoxyuridine or 3′-deoxy-3′-fluorothymidine; and of receptor expression, such as epidermal growth factor receptor, have been developed (80–85). Integration of these various tracers could provide a closer view of the biologic pathways involved in radiation responses and hence could be used to “paint” or “sculpt” the dose in various subvolumes by IMRT (86). Along this line, a few proof-of-concept studies with 18F-FDG and copper-ATSM have been reported (62,87–90). Although these studies demonstrated the feasibility of the concept of dose painting, clinical validation still remains to be undertaken.
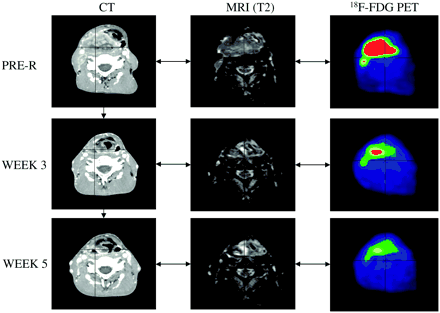
It was assumed in all of these studies that target volumes, even when defined with regard to specific biologic pathways, were homogeneous and did not vary during the course of radiation treatment. Hence, the prescribed dose was meant to be homogeneously distributed in space and time (so-called 4-dimensional homogeneous dose distribution). This situation is likely an oversimplification of the biologic reality, as tumors are known to be heterogeneous with respect to pathways of importance for radiation responses and are also known to progressively shrink (at least some of them) during treatment (91–93). For example, for HNSCC, tumor volume dramatically decreased during a 7-wk treatment (53). Thus, replanning of the radiation approach during treatment would have translated into a substantial sparing of the surrounding nontarget tissues (Fig. 5).
Patient with T4 N3 M0 squamous cell carcinoma of right piriform sinus. CT, MRI (T2-weighted fat suppression), and 18F-FDG PET were performed before treatment (PRE-R) and during wk 3 (26 Gy) and wk 5 (46 Gy) of treatment with chemoradiotherapy. Three sets of images were coregistered by use of semiautomatic tool based on isocontouring. Substantial reduction in tumor volume and metabolic activity can be observed throughout treatment.
Bentzen recently proposed the term “theragnostic” to describe the use of molecular imaging to prescribe the distribution of radiation doses in 4 dimensions—the 3 spatial dimensions plus time (94). This research topic is undoubtedly a challenging one that might revolutionize the processes of radiotherapy planning and delivery. However, before such a dream becomes reality, several issues need to be resolved. From a planning point of view, the challenge is to establish the correspondence between the PET signal intensity (or PET image segmentation) and the prescribed dose, leading to the evolution of the concept of dose painting into dose painting by numbers (95). Another challenge is to develop tools to register in space and time various images and the dose distribution acquired throughout therapy. To this end, nonrigid registration techniques will be required, as tumor or normal tissue shrinkage is expected during the course of radiotherapy (96). From a biologic point of view, the challenge is to relate a change in tracer uptake to a change in the underlying biology; this challenge requires a comprehensive biologic validation of the concept of dose painting or dose painting by numbers in experimental models. However, although much still needs to be done, it is likely that the next 5–10 y will witness some dreams becoming reality.
CONCLUSION
In summary, the introduction of PET images into the treatment planning procedure remains a challenging issue. The use of 18F-FDG PET for target volume selection should be considered within the framework of its sensitivity and specificity for various tumor types. Given the considerable ranges of accuracy of PET across different tumor types, its role will not be identical in different tumor locations. The use of PET for target volume delineation requires specific tuning of parameters, such as image acquisition, processing, and segmentation, and these parameters may vary among tumor sites. Theragnostic PET/CT with various molecular imaging probes is an emerging field; however, its clinical implementation would be premature given the paucity of clinical outcome data. In conclusion, before proper validation of the use of various PET tracers has been performed and all methodologic aspects have been fully optimized, it is reasonable to state that the use of PET for treatment planning should not be routine but should remain in the clinical research arena.
Footnotes
-
COPYRIGHT © 2006 by the Society of Nuclear Medicine, Inc.
References
- 1.↵
- 2.↵
- 3.↵
- 4.
- 5.↵
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.↵
- 12.↵
- 13.↵
- 14.
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.
- 21.
- 22.
- 23.
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.
- 29.
- 30.
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.
- 36.↵
- 37.↵
- 38.
- 39.
- 40.
- 41.
- 42.
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.
- 78.↵
- 79.↵
- 80.↵
- 81.
- 82.
- 83.
- 84.
- 85.↵
- 86.↵
- 87.↵
- 88.
- 89.
- 90.↵
- 91.↵
- 92.
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- Received for publication April 11, 2006.
- Accepted for publication September 15, 2006.