Abstract
Hürthle cell carcinoma is an uncommon and occasionally aggressive differentiated thyroid cancer associated with increased mortality compared with other differentiated thyroid malignancies. Because it generally has lower iodine avidity, 18F-FDG PET has been suggested as a more accurate imaging modality. However, there is limited information with regard to the true diagnostic accuracy and prognostic value of 18F-FDG PET in this disease. Methods: All patients with Hürthle cell thyroid cancer who underwent their first 18F-FDG PET scan between May 1996 and February 2003 were identified retrospectively. 18F-FDG PET scans were reviewed and compared with all available imaging studies, including CT, ultrasound, and radioiodine scintigraphy (RIS). Abnormal 18F-FDG uptake was assessed visually and by measuring the maximum standardized uptake value (SUVmax) of the most intense lesion. Clinical follow-up for at least 1 y or until death was required for inclusion. Results: Forty-four patients met inclusion criteria. The median follow-up was 2.9 y. There were 24 positive and 20 negative 18F-FDG PET scans with 1 false-positive and 1 false-negative study, resulting in a diagnostic sensitivity of 95.8% and a specificity of 95%. In 5 of 11 patients who had both positive CT and 18F-FDG PET findings, 18F-FDG PET revealed additional sites of disease. Furthermore, 18F-FDG PET correctly classified as negative 3 patients with false-positive CT findings. In 3 of 6 patients with positive RIS, 18F-FDG PET revealed additional sites of metastatic disease. Ten patients with positive 18F-FDG PET had negative RIS. Only 1 patient with negative 18F-FDG PET had positive RIS. The SUVmax also provided prognostic information: In a stepwise fashion, each increase in intensity by SUVmax unit was associated with a 6% increase in mortality (P < 0.001). The 5-y overall survival in patients with SUVmax < 10 was 92%; it declined to 64% in those with SUVmax > 10 (P < 0.01). Conclusion: 18F-FDG PET has excellent diagnostic accuracy in Hürthle cell thyroid cancer patients, improving on CT and RIS. Intense 18F-FDG uptake in lesions is an indicator of a poor prognosis. Our data suggest that all patients with Hürthle cell thyroid cancer should undergo 18F-FDG PET as part of their initial postoperative staging and periodically to screen for occult recurrence, particularly in patients with elevated serum thyroglobulin.
Hürthle cell thyroid cancer is an uncommon and occasionally aggressive differentiated thyroid malignancy (1), particularly when the primary tumor is widely invasive (2,3). Approximately 3.6% of thyroid cancers are of the Hürthle cell subtype (4), though there has been significant controversy among pathologists as to the exact definition of Hürthle cell thyroid cancer (5). When clearly defined histopathologically as an invasive tumor, Hürthle cell thyroid cancer has a higher incidence of distant metastasis (33%) than other differentiated thyroid cancers (range, 10%–22% in papillary or follicular thyroid cancers) (6,7). Invasive Hürthle cell thyroid cancer also has a worse prognosis: Shaha et al. reported a 20-y survival of 65%, which is significantly lower than the 87% seen in papillary thyroid cancer and 81% in follicular thyroid cancer (7). However, some studies with shorter follow-up have not shown such a striking difference in survival between Hürthle cell and follicular thyroid cancers (8,9).
For most patients with differentiated thyroid cancer and poor prognostic factors (older age, large primary tumor, extrathyroidal or vascular invasion, lymph node involvement (10)), the postsurgical management centers around therapy with radioiodine. The combination of serum thyroglobulin (Tg) measurements and radioiodine scintigraphy (RIS) is commonly used for the detection of residual or metastatic disease. However, Hürthle cell thyroid cancer presents a unique clinical dilemma because of its generally low iodine avidity (11). Indeed, the sensitivity of diagnostic RIS using 131I has been reported to be as low as 18% (12). Furthermore, medical redifferentiation therapies to increase iodine avidity have had generally disappointing results (13,14).
Serum Tg is very sensitive in detecting the presence of residual disease and the absolute Tg level may be associated with the site of metastasis (15). However, accurate localization of disease is essential in Hürthle cell thyroid cancer because surgery and external beam radiation therapy may be beneficial (16). There have been multiple attempts at imaging Hürthle cell thyroid cancer, including CT, ultrasound (US), radiolabeled somatostatin analogs, radiolabeled anticarcinoembryonic antigen antibodies, 201Tl chloride, and 99mTc-sestamibi (17–22). Although many of these modalities show increased diagnostic sensitivity compared with RIS, none of them has excellent sensitivity or specificity in detecting residual or recurrent disease.
To improve detection of Hürthle cell thyroid cancer, investigators have examined imaging of 18F-FDG (23–26). 18F-FDG PET, including semiquantitative analysis using standardized uptake values (SUVs), is of proven value in the staging and detection of recurrent disease in many malignancies (27–29), including differentiated thyroid carcinoma (30). For instance, in a group of 125 high-risk differentiated thyroid cancer patients, including 12 patients (9.6%) with Hürthle cell cancer, Wang et al. showed that a positive 18F-FDG PET scan, maximum SUV (SUVmax), and volume of 18F-FDG–avid disease all have important prognostic value (30). More recently, Robbins et al. reported further evidence that 18F-FDG PET can accurately stratify thyroid cancer patients into high and low risk of cancer-specific mortality (31).
There is limited published experience with 18F-FDG PET in the management of Hürthle cell carcinoma. The largest study to date included 17 patients along with a meta-analysis involving a total of 35 patients (26). In a group of 12 patients, Lowe et al. reported an excellent sensitivity of 92% for the detection of Hürthle cell cancer using 18F-FDG PET. However, 10 of the 12 patients had known or suspected active disease at the time of PET. In that report, the results affected patient care choices in 50% of cases (25). In the present study, we describe our own experience with 18F-FDG PET in a larger population of Hürthle cell cancer patients to verify the diagnostic accuracy of this test compared with other imaging modalities. We also investigated the prognostic value of 18F-FDG PET in this disease.
MATERIALS AND METHODS
Patients
In accordance with institutional practices, from our Memorial Sloan-Kettering Cancer Center PET database, we identified all patients with Hürthle cell thyroid cancer who underwent their first 18F-FDG PET scan between May 1996 and February 2003. All patients had previously undergone total thyroidectomy and pathologic diagnosis was confirmed by a Memorial Sloan-Kettering Cancer Center pathologist. Patients had been referred for 18F-FDG PET because of an elevated Tg, abnormal conventional imaging findings, or high-risk histopathologic findings (2). Patient charts were reviewed retrospectively for imaging and laboratory data in addition to clinical follow-up for at least 1 y or until death. Imaging and laboratory studies done within 60 d of the 18F-FDG PET were included for analysis. Imaging studies reviewed included all available CT, US, and RIS (including posttherapy scans when available). Laboratory results, with specific attention to serum Tg (15) and clinical follow-up, including biopsies from suspected sites of recurrence or metastasis when available, were all considered. An elevated Tg (≥10 ng/mL during thyroid suppression therapy), even in the absence of positive imaging, was considered positive for metastatic disease. However, a mildly elevated suppressed Tg (<10 ng/mL), which remained stable or decreased on follow-up, was considered equivocal in the presence of negative imaging. At least 1 y of clinical follow-up after 18F-FDG PET (or death within the first year with clinical follow-up until the time of death) was necessary for inclusion into the study.
PET
Patients fasted for at least 6 h before 18F-FDG injection, but liberal water intake was encouraged. Patients were instructed to sit quietly after intravenous injection of a median dose of 385 MBq (range, 337–592 MBq [10.4 mCi; range, 9.1–16 mCi]) 18F-FDG. A blood sample was drawn at the time of injection for blood glucose measurement and was confirmed to be in the acceptable range, with a median value of 91 mg/dL (range, 67–205 mg/dL).
PET studies were performed on an Advance PET (GE Healthcare; 29 patients), ECAT HR+ PET (Siemens/CTI; 3 patients), Discovery LightSpeed Plus (LS) PET/CT (GE Healthcare; 3 patients), or lutetium oxyorthosilicate (LSO) Biograph PET/CT (Siemens/CTI; 9 patients). The Advance PET was used in 2-dimensional mode and has an axial field of view (FOV) of 15.2 cm and a transaxial resolution of 4.2-mm full width at half maximum (FWHM) at the center. The ECAT HR+, using 3-dimensional acquisition, has an axial FOV of 15.5 cm and a transaxial resolution of 4.1-mm FWHM at the center. The Discovery LS PET/CT includes a 4-slice LightSpeed CT with a tube voltage of 120 kVp and a tube current of 40–85 mA, depending on patient weight. The PET component is the same as in the Advance scanner. Finally, the LSO Biograph PET/CT includes a dual-slice SOMATOM Emotion CT operated at a tube voltage of 130 kVp and a tube current of 40–85 mA, depending on patient weight. The PET component uses 3-dimensional acquisition and includes an axial FOV of 15.5 cm and a transaxial resolution of 4.5 mm at 1 cm from the center.
Images were acquired at approximately 60 min (range, 40–98 min) after intravenous injection of the radiotracer. In 25 patients, 18F-FDG PET was done from the base of the skull to the proximal thighs; in 17 patients, imaging was done from the base of the skull to midabdomen. In an additional 2 patients, who were evaluated in the early phase of clinical PET at this institution, only a limited area of interest was assessed (lower neck and chest in 1 patient; pelvis and proximal femora in 1 patient).
Images were reconstructed using ordered-subset expectation maximization. Attenuation correction was done using either rod source (GE Advance and ECAT HR+) or CT (GE Discovery LS and Siemens LSO Biograph) transmission scans. 18F-FDG PET scans were reviewed in 3 orthogonal planes and a maximum-intensity-projection image. CT, when available, was used for anatomic localization. Images were interpreted by 2 nuclear medicine physicians who were unaware of the results of correlative imaging. Findings were categorized as consistent with presence or absence of disease. A study was considered positive when focal 18F-FDG uptake was greater than background blood-pool activity and located outside of organs that show physiologic tracer uptake, such as the liver, or greater than background physiologic activity within an organ. Circular regions of interest were placed over all areas of abnormal 18F-FDG uptake and the SUVmax (corrected for body weight) was calculated. In each patient, the SUVmax of the lesion with the most intense 18F-FDG uptake was recorded.
Statistics
Categoric variables are summarized using proportions and numeric variables are presented as median and range. Survival probabilities were calculated using the Kaplan–Meier method and compared using the log-rank test (32). The association between survival times and SUVmax, age, sex, tumor extension, and cervical node status was modeled using proportional hazards regression (32). SUVmax was grouped into patients with SUVmax < 10 and ≥ 10 and survival probabilities are presented for each group. A P value of <0.05 was considered significant.
RESULTS
Forty-four patients with Hürthle cell thyroid cancer patients met study inclusion criteria and underwent their first 18F-FDG PET scan during the study interval. Studies were ordered at the discretion of the referring endocrinologist. In retrospect, 22 patients with no known disease were referred for an 18F-FDG PET scan to determine the extent of the disease. Referral was based on earlier clinical observation that 18F-FDG PET can provide diagnostic and prognostic information in patients with other entities of differentiated thyroid cancer (30). Twenty patients were referred because of clinical suspicion of disease after physical examination and Tg measurement, 1 patient for staging in light of antibodies interfering with the measurement of Tg, and 1 patient because of suggestive CT findings on follow-up. Patient characteristics are listed in Table 1.
Patient Characteristics
Tg measurements, obtained within 60 d of 18F-FDG PET, were available for 39 patients (4 of the remaining 5 patients had no sample drawn during this time interval, whereas 1 had antibodies interfering with Tg measurement). The median Tg was 6.5 ng/mL (range, 0–214,000 ng/mL).
Fifteen patients underwent CT of the neck or chest, 33 underwent RIS (24 posttherapy scans and 9 diagnostic scans), and 3 underwent US. Forty-two patients continued suppressive thyroid hormone therapy at the time of the 18F-FDG PET scan. Two patients were scanned while in the hypothyroid state (thyroid-stimulating hormone > 30 μIU/mL), both of whom had true-negative 18F-FDG PET findings.
There were 24 positive and 20 negative 18F-FDG PET scans. There was 1 false-positive and 1 false-negative scan, resulting in a sensitivity of 95.8% (95% confidence interval, 80%–100%), a specificity of 95% (76%–100%), and an overall accuracy of 95.5% (78%–100%). On long-term follow-up, 3 patients with initially negative 18F-FDG PET scans had disease recurrence at 3.2, 4.1, and 5.7 y, respectively. These recurrences all initially manifested as rising Tg levels on routine surveillance. Incidentally, in all 3 patients, follow-up 18F-FDG PET done shortly after detection of the rising Tg levels accurately localized the disease. Among patients with positive 18F-FDG PET scans, the median SUVmax was 17.6 (range, 2.1–64.9). Furthermore, when excluding the 8 patients with low-volume disease (all tumor deposits were <2 cm in maximal dimension), in whom SUVmax is likely underestimated because of volume averaging, the median SUVmax increased to 26.
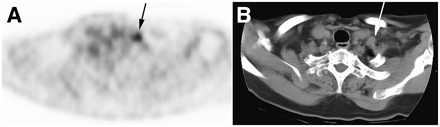
The 1 patient with a false-positive 18F-FDG PET scan had an enlarged left supraclavicular lymph node with SUVmax of 3.5. The node was also consistent with metastatic disease on a CT scan of the neck (Fig. 1). However, subsequent biopsy revealed granuloma and Mycoplasma avium grew in culture. The patient with false-negative 18F-FDG PET was shown to have iodine-avid disease in the right lung and chest wall on RIS (Fig. 2). CT of the neck and chest was also negative. After 2 annual treatments with 11.1 GBq (300 mCi) Na131I, his Tg decreased and he remains without clinical evidence of residual disease.
False-positive PET and CT. (A) Increased 18F-FDG uptake is seen in left supraclavicular lymph node (black arrow) on 18F-FDG PET. (B) Lymph node was also interpreted as malignant on dedicated CT (white arrow). Excisional biopsy showed granuloma and Mycoplasma avium grew in culture.
(A) Negative 18F-FDG PET before receiving 11.1 GBq (300 mCi) Na131I. Concurrent CT of neck and chest (not shown) was also negative. Anterior (B) and posterior (C) images from posttherapy scan 1 wk after radioiodine therapy show iodine-avid disease in neck and chest. Increased activity along the scalp was confirmed to be surface contamination. After 2 annual doses of 11.1 GBq Na131I, patient has no evidence of disease.
Fifteen patients underwent concurrent 18F-FDG PET and CT. Both imaging studies were positive in 11 of the 15 patients, but in 5 individuals 18F-FDG PET revealed additional sites of disease (4 patients with bone metastases not seen on CT and 1 patient with a cervical lymph node not seen on CT; Fig. 3). In 3 patients with true-negative PET findings, the CT was false-positive, suggesting lymphadenopathy that biopsy and follow-up revealed to be benign (Fig. 4). Finally, in 1 patient both 18F-FDG PET and CT were true-negative.
Patient with widely metastatic Hürthle cell thyroid cancer. 18F-FDG PET (A) and CT (B) both revealed pulmonary metastases, whereas left rib metastases seen on 18F-FDG PET (C) appear unremarkable on CT (D).
Contrast-enhanced diagnostic CT of the neck (A) shows a left level II cervical lymph node interpreted as consistent with metastatic disease (white arrow). (B) 18F-FDG PET shows no uptake in this node. Long-term follow-up demonstrated no evidence of disease.
Thirty-three patients underwent RIS within 60 d of 18F-FDG PET. Twenty-four of these imaging studies were posttherapy scans, which had a sensitivity of 72% in detecting at least 1 site of disease in a given patient, using the clinical follow-up and the result of conventional imaging as the gold standard. The remaining 9 studies were diagnostic scans, which had a sensitivity of 50%. Six patients had positive 18F-FDG PET and RIS scans (all posttherapy). However, in 3 of the 6 patients, 18F-FDG PET revealed multiple additional sites of uptake. Ten patients had positive 18F-FDG PET in the face of negative RIS (5 posttherapy scans and 5 diagnostic scans), including 1 false-positive 18F-FDG PET scan. Only 1 patient had false-negative 18F-FDG PET with positive posttherapy RIS. Finally, in 16 patients, 18F-FDG PET and RIS were both negative.
Only 3 patients underwent US of the neck within 60 d of 18F-FDG PET. All 3 of the patients had negative neck ultrasound and no abnormal 18F-FDG uptake in the neck. Of note, 1 of the patients had widespread pulmonary metastases on 18F-FDG PET.
During the follow-up interval, 9 patients died of thyroid cancer. All had a positive 18F-FDG PET scan. One patient with negative 18F-FDG PET findings died from complications of an unrelated surgery. Among patients dying from thyroid cancer, 2 died within 18 and 61 d after the 18F-FDG PET. Both had widespread metastatic disease. Among the remaining patients, the median follow-up after PET was 2.9 y (range, 1.2–8.8 y). Patients with SUVmax ≥ 10 had 5-y all-cause survival of 64% compared with 92% in those with SUVmax < 10 (P < 0.01; Figure 5). Each increase in 18F-FDG uptake by 1 SUVmax unit was associated with a 6% increase in mortality (P < 0.001). In univariate analysis, there was no significant difference in survival for male sex (P = 0.99), age > 45 y (P = 0.31), or degree of extrathyroidal extension (P = 0.10). In contrast, cervical node metastasis, when sampled, did show significant correlation with mortality, wherein 5-y survival in node-negative patients was 100% compared with 31% in node-positive patients (P = 0.01). However, only 15 of 44 patients underwent cervical node sampling.
Kaplan–Meier survival curve. Dashed line: Patients with SUVmax ≥ 10 have 5-y all-cause mortality of 64%. Solid line: Patients with SUVmax < 10 have 5-y all-cause mortality of 92% (P < 0.01).
DISCUSSION
Our study demonstrated that 18F-FDG PET had excellent sensitivity for disease detection and localization in our cohort of patients with Hürthle cell thyroid cancer. In addition, the intensity of 18F-FDG uptake in metastatic lesions provides important prognostic information about overall survival. Although 18F-FDG is known to be a nonspecific tracer (particularly in the face of infection or inflammation), this is mitigated by the very intense 18F-FDG uptake by most Hürthle cell thyroid cancers. Indeed, the median SUVmax per patient was 17.6 in our study and a higher SUVmax correlated strongly with an increased risk of mortality.
In a study of 12 patients with Hürthle cell thyroid cancer from the Mayo Clinic, Lowe et al. (25) reported a 92% diagnostic sensitivity for 18F-FDG PET. Here, we confirm their preliminary data in a larger patient population: Indeed, 18F-FDG PET has an excellent diagnostic accuracy in the detection of residual Hürthle cell thyroid cancer. Our results also demonstrate that 18F-FDG PET has better sensitivity and specificity than CT on a per patient basis. In 45% of patients with both positive CT and 18F-FDG PET, the 18F-FDG PET revealed additional metastatic lesions not seen on CT. Because local therapies such as surgery and external beam radiation are often pursued in metastatic Hürthle cell thyroid cancer, accurately identifying all metastatic lesions is of great clinical importance.
US was not widely used in our patient population. Indeed, only 3 patients were referred for US and all 3 had negative studies. Although US provides an excellent evaluation of the neck, few of our patients had significant morbidity or mortality from local disease in the neck. A small series examining the cause of mortality among all thyroid cancers showed that 80% of the patients died of distant metastases and only 20% died of local complications (33). Ultrasound studies of the neck were not systematically performed during follow-up of these patients. However, the correlation between cervical lymph node status at the time of thyroidectomy and mortality (though cervical nodal sampling was not done routinely) suggests that cervical nodal status may have a prognostic value in patients with Hürthle cell carcinoma. It is conceivable that ultrasound during follow-up would also add diagnostic and perhaps prognostic information. However, our retrospective study was not designed to specifically address the value of US in this patient population.
The sensitivity of RIS in our series was 65%, which is better than the 18% reported by Yen et al. (12). This is likely due, at least in part, to the fact that we included posttherapy scans when available, which have a higher sensitivity (72% in this population) than diagnostic RIS (50%). Despite this, in 50% of the positive studies, RIS underestimated the disease burden compared with 18F-FDG PET. However, though Hürthle cell thyroid cancer generally has poor iodine avidity compared with other differentiated thyroid cancers, those 50% of patients with positive iodine scans could potentially benefit from treatment with radioactive iodine. Therefore, therapy with radioactive iodine should be considered at least once in all patients (especially in light of the dearth of other effective systemic therapies). This is particularly appropriate considering the case of our patient with metastatic Hürthle cell thyroid cancer that has remained 18F-FDG PET negative and has had complete clinical response of his metastatic disease after therapy with radioactive iodine.
Even among high-risk patients with suspected or known metastatic disease, 18F-FDG PET had a striking contribution over conventional imaging modalities. We found that an SUVmax of 10 distinguishes between subsets of patients with relatively good prognosis and those with poor prognosis. We have chosen to use an SUVmax cutoff of 10, building on the work of Wang et al., who showed that this SUV number differentiates between good and poor prognosis in a larger population of all differentiated thyroid cancers (30). Furthermore, age, sex, tumor extension, and cervical node status were examined, as their prognostic significance had been assessed previously in a series of 555 patients with Hürthle cell thyroid cancer (8). We found that age, sex, and primary tumor extension were not correlated with survival, whereas cervical node status did correlate with survival.
Three of 20 patients with negative 18F-FDG PET scans did develop recurrent disease an average of 4.1 y after their initial 18F-FDG PET. However, these 3 patients all had positive 18F-FDG PET findings shortly after recurrence was suspected because of rising Tg levels. No foreseeable imaging modality can detect microscopic disease, but these data suggest that 18F-FDG PET may detect disease sooner than other imaging modalities.
We have reported the SUVmax of only the most intense lesion in each patient because it is likely that prognosis and therapeutic options are determined by the most aggressive disease in a given patient. Furthermore, the most intense lesions tend to be the largest lesions, which minimizes the effects of volume averaging. Additionally, as each patient may have a spectrum of disease, reporting the SUVmax for each hypermetabolic lesion would likely cause more overlap among patients, possibly obscuring the significance of our findings.
Our data are limited by the fact that this is a retrospective study. There may have been referral bias, particularly among the earliest patients. On the basis of the initial clinical data, all patients with Hürthle cell thyroid cancer now undergo at least one 18F-FDG PET study shortly after thyroidectomy and before postsurgical radioiodine therapy. This regimen was followed in about half of the study population, whereas in earlier cases 18F-FDG PET was done at the discretion of the referring endocrinologist. Furthermore, it would be preferable to have all correlative imaging and laboratory tests performed in a narrower time range than 60 d of 18F-FDG PET. However, because of scheduling constraints, many patients could not have these tests completed more expeditiously. We initially hoped to discuss the impact of the 18F-FDG PET studies on subsequent patient care decisions. However, most patients returned to their referring physician after all imaging and laboratory studies were done. Therefore, the referring physician's note after the 18F-FDG PET study did not specifically address the impact of 18F-FDG PET but, rather, synthesized all available data. We decided that a retrospective analysis of the likely impact of the 18F-FDG PET scan would require too much inference. Instead, we chose to concentrate on the accuracy of 18F-FDG PET and its prognostic implications in this patient population. Ideally, the true prognostic value and impact of 18F-FDG PET in Hürthle cell thyroid cancer should be assessed in a prospective study. Such a study would require long-term follow-up because of the protracted time course of many thyroid cancers.
CONCLUSION
Hürthle cell thyroid cancer tends to be 18F-FDG avid with very intense, conspicuous uptake on 18F-FDG PET. 18F-FDG PET has excellent diagnostic accuracy in Hürthle cell thyroid cancer and often provides additional information over conventional imaging. In patients with a clinical suspicion of recurrent disease because of rising Tg levels, 18F-FDG PET is likely the most accurate imaging test in localizing metastatic disease. Finally, 18F-FDG PET conveys important prognostic information that may potentially alter patient management. We suggest that all patients with Hürthle cell thyroid cancer should undergo 18F-FDG PET as part of their initial postoperative staging and during follow-up when there is a rising Tg level or other clinical suspicion of recurrent disease.
Footnotes
-
COPYRIGHT © 2006 by the Society of Nuclear Medicine, Inc.
References
- Received for publication March 21, 2006.
- Accepted for publication May 8, 2006.