Abstract
Malignant melanoma of the skin is one of the most lethal cancers. The disease may spread either locally or regionally and to distant sites through predictable or unpredictable metastatic pathways. Accurate staging and restaging of disease are required for appropriate treatment decision making. Routine protocols based on clinical examinations and traditional radiologic evaluations are not cost-effective for the detection of systemic disease. In the last decade, nuclear medicine techniques, such as lymphoscintigraphy-directed lymphatic mapping and sentinel lymphadenectomy and 18F-FDG PET, have played key roles in nodal and distant staging of melanoma. More recently, anatomic–functional imaging has been improved with the development of integrated PET/CT devices or combined SPECT/CT systems. 18F-FDG–sensitive intraoperative probes have been specifically designed to detect small nodal and visceral metastases from melanoma and may become important tools for the cancer surgeon. In this article, we review the role of nuclear medicine in the assessment of malignant melanoma.
Cutaneous malignant melanoma (CMM) is one of the most deadly cancers (1). Although the disease accounts for only about 4% of skin cancer–related cases, it is responsible for about 79% of skin cancer deaths. In the United States, the incidence of CMM is on the rise, and the disease-related mortality rate has dramatically increased by 50% since 1973 (2).
Early diagnosis of this highly malignant skin cancer, along with accurate staging of disease extent, is therefore essential for appropriate treatment decision making and, in turn, may give patients with melanoma the best chances of prolonged survival (3–5). From this perspective, several key variables have been identified in the natural history of CMM; these include Breslow thickness, Clark level, ulceration, tumor location, growth pattern, histological subtype, patient age and sex, and tumor status of regional lymph nodes (6). Among these clinical and pathological parameters, the histological and oncogenic status of the first node(s) draining the primary tumor, also called sentinel lymph node(s) (SLN), was found to be the most powerful prognostic factor in early-stage melanoma patients (7,8). Logically, the American Joint Committee on Cancer (AJCC) recently incorporated the histological status of SLN into its latest staging system for CMM, as shown in Tables 1 and 2 (9).
TNM Staging System for CMM
AJCC Staging System for CMM
In addition to the propensity of melanoma tumor cells to progress in an orderly fashion from the primary tumor to the regional lymph nodes, the importance of hematogenous factors that may be the cause of extranodal metastases early in the course of disease also should be considered (10–12). Local and distant lesions from the primary site may be discovered in patients with no regional lymph node disease (13). Routine protocols, including physical examination and conventional imaging techniques, such as chest radiography, ultrasonography, CT, and MRI, are of limited value for identifying systemic melanoma, especially in asymptomatic patients with metastatic SLNs (14). A highly sensitive whole-body imaging procedure, such as PET with 18F-FDG as a marker of malignancy, is needed for the proper staging and restaging of high-risk melanomas (15).
In today's clinical practice, PET/CT scanners allow for the simultaneous assessment of both metabolic and anatomic characteristics of the primary tumor and its potential local, regional, and distant extension (16). Similarly, more accurate localization of SLNs before surgery is achievable with the new hybrid SPECT/CT systems (17). In translational research, 18F-FDG–sensitive intraoperative probes are being designed specifically to optimize the detection of melanoma tumor sites in vivo (18).
In this continuing education article, we review the clinical contribution of current nuclear medicine technologies to the optimal management of CMM. To this end, we have defined several learning objectives to best address the topic.
PATTERNS OF DISSEMINATION
Understanding the patterns of dissemination of CMM is essential for defining the most effective imaging work-up schedule (10,11). In the natural course of CMM, 3 different metastatic pathways have been well identified; these include local extension via satellite metastases (metastatic nodules within 2 cm from the primary site) or in-transit metastases (metastatic lymphatic drainage between the primary site and the first regional lymph node basin), regional extension to regional lymph nodes, and distant extension to visceral and nonvisceral organs. Patterns of dissemination are shown in Figure 1.
According to orderly progression of malignant melanoma, main pathway for tumor spread involves regional lymph nodes draining primary tumor. Melanoma tumor cells may skip first nodal station, resulting in either local (satellite or in-transit) metastases or distant metastases.
Regardless of the depth of the primary tumor, most patients who have melanoma classified as AJCC clinical stage I or II (or any T N0 M0) at the time of diagnosis will ultimately develop regional lymph node metastases as the first presentation of disease spread (10). In a few patients, nodal disease may occur without simultaneous distant metastases; this subset of patients is the most likely to benefit from lymphatic mapping and sentinel lymphadenectomy (LM/SL), complete lymphadenectomy, or adjuvant immunotherapy (7,8). Most patients with lymph node metastases develop extranodal disease, such as satellite or in-transit metastases or distant metastases (11). Recent data highlighted the simultaneous occurrence of satellite or in-transit metastases and distant metastases with nodal metastases, suggesting that the hematogenous spread and lymphatic spread pathways may occur concomitantly (11–13). On the basis of clinical and radiologic findings, distant metastases from CMM may affect all organs at variable frequencies: skin (13%–38%), distant lymph nodes (5%–34%), lungs (18%–36%), pleura (3%), liver (14%–20%), central nervous system (2%–20%), bone (4%–17%), gastrointestinal tract (1%–8%), heart (<1%), pancreas (3%), adrenal glands (1%–11%), kidneys (<1%), and thyroid gland (<1%). Rates of metastases are much higher in autopsy studies (11,13,19–25). Sites and rates of distant metastases from CMM are reported in Figure 2.
Tumor dissemination in CMM involves both lymphatic and hematogenous pathways. During course of disease, any organ may be affected, including skin and subcutaneous, nodal, and visceral sites.
Early Stages of Disease: AJCC Stages I and II
LM/SL
In early-stage melanoma (AJCC stage I or II), LM/SL has become the standard of care for the staging of regional lymph nodes (26). The functional routes of lymphatic drainage revealed by LM/SL may contradict Sappey anatomic guidelines (27,28). Hence, in patients with localized CMM, LM/SL enables surgeons to assess the histological status of regional lymph node basins through predictable and unpredictable SLNs.
Compelling evidence from the literature indicates that LM/SL is the procedure of choice for the detection of occult regional lymph nodes metastases, particularly in patients presenting with an intermediate Breslow thickness (1–4 mm) (7,8). Typically, the LM/SL technique includes preoperative lymphoscintigraphy with dynamic sequences and static views and then intraoperative detection with a blue dye or a hand-held γ-probe (26,29,30).
Preoperative lymphoscintigraphy is the first step in the lymphatic mapping procedure and is considered a “road map” guiding the surgeon, especially for localizing unpredictable lymphatic drainage patterns (27–29). Ideally, the lymphoscintigraphy report should include the following information:
Identification of all draining hot nodes, because not all hot nodes are SLNs, and the SLN is not necessarily the hottest node (31).
Identification of all drainage basins. Multiple hot nodes that belong to a single basin should be differentiated from multiple hot nodes that belong to separate basins and need to be identified at surgery and examined (32,33).
Identification of unpredictable SLNs, including in-transit lymph nodes (i.e., lymph nodes located between the primary tumor site and a drainage basin) and aberrant lymph nodes (i.e., lymph nodes located outside a standard drainage basin). The incidence of metastatic in-transit or aberrant nodes in melanoma has been reported to vary between 14% and 22% (34). The highest incidence of in-transit SLNs occurs in posterior trunk melanoma, most often at the triangular intermuscular space lateral to the scapula, followed by melanoma in the anterior trunk, the head and neck, the upper limbs, and the lower limbs (27,28,33,35).
Confirmation of true nodal versus nonnodal sites of uptake, such as skin folds, radiopharmaceutical contamination, and lymphangioma, which are common causes of false-positive results on conventional planar images (36). This confirmation relies on various technical factors, including the choice of radiocolloids, the appropriate mode of injection, the performance of early dynamic acquisition and of multiprojection planar imaging of all potential drainage basins, and the use of body-outlining methods to facilitate the topographic localization of scintigraphy findings. The latter can be achieved with a radioactive source (i.e., a hand-held point source, a 57Co flood source for simultaneous transmission imaging) or a coregistration using the scintigraphy and CT data (29,30,35).
LM/SL and SPECT/CT
The recent introduction of hybrid systems consisting of CT (low dose or diagnostic) and a γ-camera combined in a single gantry enables transmission (CT) and emission (SPECT) procedures to be performed during the same study, with automatic fusion of images from both modalities. External or internal landmarks are not needed for hybrid SPECT/CT imaging. The radiation dose added to the scintigraphic procedure when low-dose CT is performed ranges from 1.3 mGy at the center to 5 mGy at the surface of the body. However, it may reach 20 mGy when fully diagnostic CT is performed (17,36).
SPECT/CT acquisition is performed after dynamic and static planar images are obtained. SPECT is performed first, and then low-dose CT is performed. With a hybrid system, the quality of SPECT images is improved by the use of CT maps for attenuation correction. The anatomic localization of lesions is more accurate, as it is determined by the 3-dimensional data of SPECT and by the anatomic details of CT (Fig. 3). Images of the relevant fused SPECT/CT slices in the 3 planes are given to the surgeon before surgery. The technical details for a SPECT/CT protocol in patients who have melanoma and are undergoing LM/SL are provided in Table 3.
(A) On preoperative lymphoscintigraphy images, planar views with (right) and without (left) 57Co flood revealed 3 hot nodes in both inguinal regions (arrows). (B) On SPECT/CT images, 5 hot nodes (from left to right) were found to be bilateral superficial and deep inguinal node basins and right external iliac pelvic node (arrows).
SPECT/CT Protocol for LM/SL in CMM
The clinical information added by SPECT/CT may be valuable when one is reporting lymphoscintigraphy findings in the 4 categories mentioned earlier, particularly in patients with melanoma of body parts with complex lymphatic drainage systems, such as the head and neck and the posterior trunk (35,37). For instance, SPECT/CT was shown to identify hot nodes missed on planar images, including nodes invaded by metastases in 43% cases of primary melanoma located in the head and neck or trunk region. These SLNs identified by SPECT/CT were nodes hidden by injection site scattered radiation, deeply located nodes, or in-transit nodes. Multiple drainage basins were clearly identified in 50% of patients with trunk melanoma and in 33% patients with head and neck malignancy.
Overall, the use of SPECT/CT for SLN detection is still in its infancy. However, the initial results are promising for the better surgical management of melanoma. This anatomic–functional technique enables more accurate nodal staging and has the potential for reducing morbidity from primary melanomas of the trunk and the head and neck. Further multicenter studies aimed at evaluating the added value of SPECT/CT for LM/SL will help provide more experience in this new field of investigation.
Whole-Body 18F-FDG PET
Cumulative data from 11 referenced series including 610 patients with early-stage melanoma showed that whole-body 18F-FDG PET had a low mean sensitivity of 17.3% (0%–40%) for detecting SLN metastases (38–48). Literature data are summarized in Table 4. In line with these clinical data, a simulation study confirmed that current PET devices with a spatial resolution of about 4–6 mm have sensitivities of less than 50% for the detection of SLN metastases from melanoma (49). Previous data showed that the sensitivity of 18F-FDG PET varied significantly with the sizes of lymph node metastases (50). PET sensitivity was only 23% for lymph nodes of less than 5 mm and increased to 83% and 100% for lymph nodes of 6–10 mm and of greater than 10 mm, respectively. More recent data showed that the mean aggregated SLN tumor volume was 4.3 mm3 (0.07–523 mm3), whereas the mean volume identified by mid-end PET scanners was about 65 mm3 for a spatial resolution approaching 4 mm (51). Accordingly, the minimal threshold to reach 90% sensitivity with 18F-FDG PET was estimated to be 78 mm3. Below this tumor volume (i.e., <78 mm3), PET sensitivity dropped to 14% (52). Therefore, regardless of PET protocols and equipment, metabolic imaging is inaccurate for the detection of small nodal metastases from melanoma, which are most often only 1–2 mm in size.
18F-FDG PET Versus LM/SL for Detection of Regional Lymph Node Metastases from Melanoma
LM/SL and Whole-Body 18F-FDG PET
In view of the complex metastatic behavior of CMM, the occurrence of metastases bypassing the first regional nodal basin should be considered even in early stages of disease (6,53). In a retrospective series of 13 patients who had melanoma and who underwent both LM/SL and whole-body 18F-FDG PET within 4 mo, Quan et al. reported 38% discordant findings at initial staging (5/13 patients) (54). Among them, 2 patients with negative LM/SL had distant metastases detected by 18F-FDG PET, whereas 2 patients with SLN metastases had negative 18F-FDG PET. In 1 patient with no SLN identified, PET detected metastases (54). Similarly, Vereecken et al. recently emphasized the importance of extensive initial staging for intermediate or high-risk melanomas (Breslow thickness of ≥1 mm, regression, and ulceration) (47). In their series including 43 patients, extensive staging based on LM/SL and CT allowed early treatment in 12 patients (28%). Although the role of 18F-FDG PET alone was essentially limited to the detection of SLN metastases, newly integrated whole-body 18F-FDG PET/CT may prove to be clinically more cost-effective for a selected category of early-stage melanomas. Further studies are necessary to identify patients who have early-stage melanoma, who have negative LM/SL results but who are at a higher risk of distant disease, and who may best benefit from the staging capabilities of whole-body 18F-FDG PET (PET/CT). Molecular biology techniques and genetic profiling with reverse transcription–polymerase chain reaction and DNA signatures should help identify phenotypes responsible for disease progression from the primary tumor to SLNs and distant sites (54–57).
Overall, in the initial work-up of patients with AJCC stage I or II melanoma, LM/SL combined with SPECT/CT remains the procedure of choice for regional nodal staging, instead of whole-body 18F-FDG PET, because these patients have a low risk of distant metastases even with occult regional nodal disease. In particular clinical circumstances, such as when the pretest likelihood of distant metastases is no longer negligible, as in higher-risk melanomas (i.e., melanomas of the trunk and upper arms and those with a Breslow thickness of >4 mm, ulceration, and a high mitotic rate), one may reasonably consider the potential utility of whole-body 18F-FDG PET (or, better yet, PET/CT) in addition to LM/SL.
Advanced Stages of Disease: AJCC Stages III and IV
Conventional Staging
Malignant melanoma of the skin can spread widely and unpredictably throughout the body. Median survival after the appearance of distant metastases is approximately 6 mo (6). Patients with AJCC stage III or IV melanoma, including those with clinically identified metastases that have been surgically resected, are at high risk for recurrent disease. Accurate staging is therefore important to determine the usefulness of potentially curative surgery or radiation therapy in patients with recurrent disease. Guidelines for the clinical and radiologic follow-up of these patients are sparse (4). Most often, follow-up regimens are tailored to each patient on the basis of the sites involved and the completeness of surgery.
The value of a comprehensive clinical history and physical examination should not be underestimated for the detection of recurrent disease. In routine practice, these “low-tech tools” provide the foundation for all other diagnostic studies, including measurement of the levels of serum lactate dehydrogenase, serum S-100 protein, and C-reactive protein, chest radiography, and chest CT combined with CT of the abdomen and pelvis, to completely assess the chest, mediastinum, abdomen, and pelvis for recurrent disease (58–62). Nonetheless, conventional staging investigations (ultrasonography, CT of the chest, CT of the abdomen, and brain MRI, with or without bone scanning) have limited sensitivity and specificity for the detection of melanoma metastases, particularly in asymptomatic patients (14,63,64). In studies of patients with stage II–IV melanoma, these routine imaging techniques have a sensitivity of 57%–81% and a specificity of 45%–87% for single melanoma lesions (65–67). In a further study of 347 patients with clinical stage III melanoma, CT scans identified twice as many false-positive as true-positive melanoma lesions (68).
Whole-Body 18F-FDG PET
Whole-body 18F-FDG PET is the newest addition to the armamentarium of the clinician searching for metastatic disease. PET has been proven to be superior to standard diagnostic procedures for the detection of distant metastases. In studies and meta-analyses reported in the literature, the sensitivity, specificity, and accuracy of 18F-FDG PET for detecting recurrent melanoma ranged from 70% to 100% (15,65–67,69–73). Accordingly, 18F-FDG PET was found to be particularly sensitive and specific for detecting soft-tissue and lymph nodes metastases that could not be assessed by clinical examination and that were not demonstrated by CT. 18F-FDG PET also was shown to detect disease up to 6 mo earlier than conventional techniques (15,66,74). In studies directly comparing 18F-FDG PET with conventional imaging in patients with recurrent melanoma, 18F-FDG PET showed superior accuracy for detecting both local–regional and distant metastases (Fig. 4) (69,70). Most lesions missed by 18F-FDG PET are typically less than 1 cm in diameter and are mainly pulmonary and hepatic or located in the brain (75). The vast majority of these false-negative results can be detected by CT or MRI for brain metastases. As such, whole-body 18F-FDG PET should complement rather than replace CT or MRI in patients with suspected recurrent disease. Most lesions missed by CT are located in the abdomen, suggesting that 18F-FDG PET can especially assist in staging for this region. 18F-FDG PET also has been compared with other staging techniques for metastatic melanoma. In a series of 121 patients with metastatic melanoma, in which 18F-FDG PET was compared with 67Ga scintigraphy, PET was more accurate and provided incremental and clinically important information in 10% of patients and did so at a lower cost (76). 18F-FDG PET is therefore the principal staging technique for the assessment of recurrent melanoma, usually in conjunction with CT of the thorax for pulmonary lesions and MRI of the brain for possible brain metastases.
(A) A 45-y-old-man with history of melanoma of right neck presented with palpable lymph nodes in right neck. 18F-FDG PET showed extensive metastatic disease. (B) A 73-y-old-man with history of melanoma of left scalp presented with undetermined lung nodule in right lower lobe. 18F-FDG PET scan showed single metastasis in right lung; lesion was suitable for resection. (C) A 61-y-old-man with history of melanoma on back presented with posterior neck lesion. 18F-FDG PET scan showed only right axillary mass and no other sites of metastatic disease.
Unsuspected additional primary tumors also may be detected by use of 18F-FDG PET in the follow-up of patients with metastatic melanoma. In a series of 92 patients with primarily AJCC stage IV melanoma, 7 new primary tumors were detected by 18F-FDG PET, emphasizing the importance of considering differential diagnoses and obtaining a tissue diagnosis, particularly at the time of the first relapse (70).
Information on the direct impact of 18F-FDG PET on the clinical management of melanoma strongly supports its role in the assessment of potential recurrent disease. In the majority of cases, whole-body 18F-FDG PET alters the decision to perform surgery for recurrent disease. Surgery can be curative for stage III disease and is the only therapy that influences survival in patients with stage IV disease (77). Up to one quarter of patients with metastatic disease are candidates for potentially curative surgical resection, and 20% of patients for whom curative resection is achieved become long-term survivors (78). Retrospective studies of patients with predominantly stage III and IV disease suggested that 18F-FDG PET results influenced disease management for 22%–49% of patients (67,69,79,80). In 2 prospective studies, 18F-FDG PET results changed disease management 15% of the time in a series of 95 patients with stage III disease and contributed to a change in therapy 40% of the time in a series of 58 patients with suspected recurrent melanoma (81,82). In a recent study of patients with primarily AJCC stage IV disease, the impact of 18F-FDG PET scans on disease management was well documented in 40 of 126 patients (32%), particularly in assisting in the selection of patients for surgery (70). However, 18F-FDG PET can miss small-volume disease or micrometastatic disease, as evidenced by some false-negative 18F-FDG PET studies and by the number of patients who relapsed soon after surgery. Therefore, 18F-FDG PET can help to guide the appropriate use of surgery in this patient population but may not guarantee a long-term favorable outcome after surgery.
Whole-body 18F-FDG PET also may play a useful role in the monitoring of metastatic melanoma. This role may be particularly relevant in patients who have unresectable regional or distant metastatic disease and who are included in immunotherapy or chemotherapy protocols. At this stage of advanced disease, chemotherapy has never been shown to provide an overall survival benefit, although it induces occasional transient disease regression. Immunotherapy has been shown to have more notable effects, with durable responses being achieved with high-dose interleukin 2 and interferon. These results led to the approval of the former for the treatment of metastatic disease and to the evaluation of the latter as an adjuvant therapy (83). However, the apparent inability of any agent tested to improve overall survival in patients with melanoma urgently supports the need for alternative therapies. As reported for other tumor types, 18F-FDG PET has been shown to accurately detect early metabolic responses to conventional and experimental therapies in patients with metastatic melanoma (Fig. 4). The timely incorporation of whole-body 18F-FDG PET into clinical trial protocols may help to improve the efficacy of treatment regimens, including dosage and scheduling optimization (84).
Whole-Body 18F-FDG PET/CT
The development of combined PET/CT scanners has dramatically changed the approach to PET image interpretation. The seamless integration of anatomic (CT) and metabolic (PET) images allows for the more accurate determination of abnormal sites and their clinical relevance (85,86). The superior accuracy of PET/CT over that of PET in the assessment of metastatic melanoma has already been reported (16,87). PET/CT has a significant impact on the interpretation of suspected metastatic lesions (Fig. 5). Also, the false-negative and false-positive rates of 18F-FDG PET can be reduced through the use of PET/CT, particularly by providing clarity for normal 18F-FDG uptake variants (88–91). In a recent study including 250 patients with melanoma (AJCC stages I–IV), PET/CT was found to be significantly more accurate than PET alone and CT alone for the staging of visceral and nonvisceral metastases. Noteworthy was that the CT portion of PET/CT was particularly helpful for the detection of lung metastases, which are often missed by PET alone. These data also showed an incremental treatment impact of PET/CT in the settings of restaging and therapy control in patients with melanoma (92). Figure 6 shows the added value of PET/CT for differentiating physiologic from pathologic 18F-FDG uptake patterns.
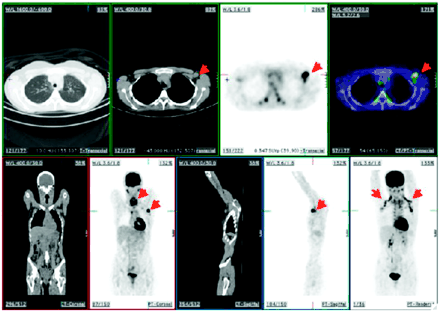
A 70 y-old-man with history of malignant melanoma presented with right axillary lymph node enlargement. (A) Coronal 18F-FDG PET scan showed right axillary disease (arrow). (B) CT scan. (C) Transaxial 18F-FDG PET scan. (D) Coregistered PET/CT images showed uptake of 18F-FDG in right axillary lymph node (arrow).
A 25-y-old-woman with left axillary melanoma on biopsy was referred for PET/CT scan. 18F-FDG PET scans showed 18F-FDG uptake in left axillary node (arrow). Uptake in neck and costovertebral regions was consistent with physiologic muscle and fat uptake (arrow). Thyroid uptake also was noted and was consistent with mild inflammatory change only (arrow). Patient was classified as having AJCC stage IIIB disease.
The precise anatomic localization of sites of abnormal 18F-FDG uptake enables the identification of patients who are suitable for surgery as well as the localization of isolated metastases (Fig. 7) (16,71,88). PET/CT is also helpful for the staging of unusual sites of primary melanoma, such as ocular melanoma and vaginal melanoma (93–95). In addition, PET/CT can identify incidental metastatic melanoma in patients with other malignancies, although this is an unusual finding (90).
A 45-y-old-woman with metastatic melanoma was referred for evaluation of paraaortic lymph nodes. (A) Whole-body PET in rotating view showed focal area of 18F-FDG uptake in right upper abdomen (arrow); this uptake was confirmed on transaxial slices from CT scan (B) and PET scan (C). (D) Fused PET/CT images localized 18F-FDG–avid foci in second part of duodenum, consistent with metastatic deposit (arrows). No abnormality of paraaortic lymph nodes was observed.
PET/CT has been shown to play a potential role in patients undergoing clinical trials for new therapeutics. Further assessment of this technology, including the ability to integrate PET/CT data in therapy planning, is important for the management of melanoma in the future. Table 5 summarizes the added value of PET/CT for the management of malignant melanoma.
Added Value of Integrated PET/CT for Management of CMM
In an effort to standardize the acquisition procedure for integrated PET/CT scanners, a joint working group involving the American College of Radiology, the Society of Nuclear Medicine, and the Society of Computed Body Tomography and Magnetic Resonance recently recommended the inclusion of images of both upper and lower extremities for patients with malignant melanomas that involve the extremities and images of the head for patients with known or suspected scalp involvement (96).
18F-FDG–Sensitive Probe
The role of surgery in recurrent or metastatic cancer is being redefined. With the advance of tools of early diagnosis and improvements in systemic therapy, metastasectomy and cytoreductive surgical techniques in selected cases are considered viable management options. Retrospective data suggested improved survival in patients who had stage IV melanoma and who underwent complete resection of limited metastatic disease followed by immunotherapy (97).
In the era of 18F-FDG PET and increasingly of PET/CT, many clinical studies have attested to the sensitivity and accuracy of metabolic imaging for the evaluation of malignant melanoma (15,65–67,69–73,92). The photons that are released during the annihilation process and that are detected for imaging also can be captured by a hand-held probe designed to process high-energy photons. A hand-held 18F-FDG–sensitive intraoperative probe can be a valuable adjunct for the surgical localization of PET-positive tumors (98).
A pilot study performed at the John Wayne Cancer Institute showed the feasibility of a hand-held 18F-FDG–sensitive intraoperative probe in metastatic or recurrent melanoma (18). All patients selected for PET probe–guided diagnostic exploratory surgery had a positive 18F-FDG PET scan, which revealed abnormal focal uptake during a comprehensive work-up without any prior cancer diagnosis. The PET probe detected all 18F-FDG PET–positive lesions. The smallest detectable lesion was 0.5 cm. In addition, the PET probe was instrumental in the localization of lesions that were not seen on the preoperative imaging study (retroperitoneal foci) or not immediately apparent at surgical exploration, particularly in a previously explored field (neck, axilla, and abdomen). The probe also was helpful for detecting nonpalpable lesions (neck, axilla, lungs, and soft tissue). Reports from other centers also highlighted the potential of an intraoperative PET probe for the detection of occult recurrent melanoma after 18F-FDG PET or PET/CT (99,100).
At present, 18F-FDG imaging represents the standard for functional and biologic imaging in oncology. However, areas of physiologic 18F-FDG uptake have been shown to considerably limit the detection efficacy of an intraoperative PET probe. New PET radiopharmaceuticals, particularly fluorinated agents, may yield better in situ tumor-to-background ratio profiles than 18F-FDG for a given lesion. For instance, Cobben et al. reported the early results of 18F-fluoro-l-thymidine (18F-FLT) imaging in patients with melanoma (101). Unlike 18F-FDG, 18F-FLT does not accumulate in the brain. This specific pattern of uptake would make 18F-FLT a better PET probe agent for explorations of the head and neck. The same agent, however, would be a poor choice for explorations of hepatic lesions because of the high physiologic background related to 18F-FLT metabolism.
In summary, a PET probe with further refinements in design and technical performance might prove to be a useful tool in the surgical management of recurrent or metastatic disease (100). The surgical indications of a PET probe will be individually determined on the basis of tumor and patient characteristics as well as the patterns of biodistribution of the selected PET radiopharmaceuticals. Until now, the clinical benefit of metastasectomy in melanoma or other cancer types has not been substantiated by controlled studies. The real clinical value of a PET probe can be supported only if such benefit can be demonstrated in clinical trials. Table 6 summarizes the role of nuclear medicine procedures in the management of CMM.
Nuclear Medicine Platform for Management of Malignant Melanoma
CONCLUSION
CMM is one of the most aggressive cancers. The coexistence of various metastatic pathways based on lymphangiogenesis and angiogenesis requires a comprehensive assessment of disease before and after treatment.
Nuclear medicine–based techniques, such as LM/SL, 18F-FDG PET and, more recently, anatomic–functional imaging with hybrid devices, such as PET/CT and SPECT/CT, play a pivotal role in the overall management of CMM. To this end, each procedure should be performed by taking into account the natural course of the disease. A large body of evidence has already shown the utility of LM/SL in early stages of disease (AJCC stages I and II), whereas 18F-FDG PET is preferably indicated in advanced stages of disease (AJCC stages III and IV). In addition, metabolic imaging with 18F-FDG has been proven to be the technique of choice for the posttherapy monitoring of patients with melanoma.
The recent introduction of combined PET/CT devices will significantly improve the diagnostic accuracy of metabolic imaging in the evaluation of patients with melanoma. Initial data also have highlighted the added value of hybrid SPECT/CT for the more accurate localization of SLNs with LM/SL, particularly in patients with head and neck melanomas and trunk melanomas. Along with optimized intraoperative 18F-sensitive probes, these technological improvements may open new avenues for image-guided therapy as well as radio-guided surgery.
Additional prospective studies to assess the performance of LM/SL plus 18F-FDG PET (PET/CT) in various categories of patients with different pretest likelihoods of metastases based on clinical, pathological, and molecular criteria need to be performed. Multicenter studies will determine the added value of SPECT/CT in patients undergoing LM/SL. Last but not least, cost-effective studies aimed at assessing the clinical added value of 18F-FDG PET (PET/CT) in the follow-up surveillance of patients with melanoma are urgently needed to provide clinicians with a valuable imaging algorithm.
Footnotes
-
↵* NOTE: FOR CE CREDIT, YOU CAN ACCESS THIS ACTIVITY THROUGH THE SNM WEB SITE (http://www.snm.org/ce_online) THROUGH JUNE 2007.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.
- 56.
- 57.↵
- 58.↵
- 59.
- 60.
- 61.
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- Received for publication December 28, 2005.
- Accepted for publication April 20, 2006.