Abstract
All lanthanides have similar chemical properties regarding labeling. Therefore, radiolanthanides that have been used for therapy, such as 153Sm and 177Lu, might easily be replaced with other radiolanthanides. The aim of this work was to investigate the suitability of electron- and positron-emitting radiolanthanides for radionuclide therapy with reference to dosimetry and production possibilities. Methods: Radiolanthanides with half-lives of 1 h to 15 d, stable or long-lived daughters, and limited photon emission were selected. The ratio of the absorbed dose rate to the tumors and the normal tissue (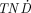 ) was calculated for different tumor sizes and compared with the
) was calculated for different tumor sizes and compared with the 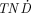 values for 90Y and 131I. The normal tissue and tumors were simulated as an ellipsoid and spheres, respectively. The
values for 90Y and 131I. The normal tissue and tumors were simulated as an ellipsoid and spheres, respectively. The  values depend on the physical parameters of the radionuclides, the tumor size, and the ratio between the activity concentrations in the tumor and normal tissue (TNC). Results: 153Sm, 161Tb, 169Er, 175Yb, and 177Lu had the highest
values depend on the physical parameters of the radionuclides, the tumor size, and the ratio between the activity concentrations in the tumor and normal tissue (TNC). Results: 153Sm, 161Tb, 169Er, 175Yb, and 177Lu had the highest  values for most of the tumor sizes studied. Among these radiolanthanides, 161Tb and 177Lu are the only ones that can be produced no-carrier-added (nca) and with high specific activities. The Auger-electron emitters 161Ho and 167Tm had high
values for most of the tumor sizes studied. Among these radiolanthanides, 161Tb and 177Lu are the only ones that can be produced no-carrier-added (nca) and with high specific activities. The Auger-electron emitters 161Ho and 167Tm had high  values for tumors weighing less than 1 mg and can be produced nca and with high specific activities. 142Pr, 145Pr, and 166Ho showed
values for tumors weighing less than 1 mg and can be produced nca and with high specific activities. 142Pr, 145Pr, and 166Ho showed 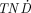 values similar to those of 90Y. 166Ho is generator produced and can be obtained nca and at high specific activities. 143Pr, 149Pm, 150Eu, 159Gd, 165Dy, 176mLu, and 179Lu had higher
values similar to those of 90Y. 166Ho is generator produced and can be obtained nca and at high specific activities. 143Pr, 149Pm, 150Eu, 159Gd, 165Dy, 176mLu, and 179Lu had higher  values than did 90Y for all tumor sizes studied, but only 149Pm can be produced nca and at high specific activities. The other electron-emitting radiolanthanides and the positron-emitting radiolanthanides showed low
values than did 90Y for all tumor sizes studied, but only 149Pm can be produced nca and at high specific activities. The other electron-emitting radiolanthanides and the positron-emitting radiolanthanides showed low 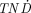 values for all tumor sizes because of the high photon contribution. Conclusion: The low-energy electron emitters 161Tb, 177Lu, and 167Tm might be suitable for radionuclide therapy. The Auger-electron emitter 161Ho might not be suitable for systemic radionuclide therapy (intravenous injection) because of its short half-life but might be suitable for local therapy (e.g., in body cavities). If higher electron energy is needed, 149Pm or 166Ho might be suitable for radionuclide therapy.
values for all tumor sizes because of the high photon contribution. Conclusion: The low-energy electron emitters 161Tb, 177Lu, and 167Tm might be suitable for radionuclide therapy. The Auger-electron emitter 161Ho might not be suitable for systemic radionuclide therapy (intravenous injection) because of its short half-life but might be suitable for local therapy (e.g., in body cavities). If higher electron energy is needed, 149Pm or 166Ho might be suitable for radionuclide therapy.
The 14 elements with atomic numbers between 58 and 71 are called lanthanides. These metal ions all have about the same radius, which makes them chemically similar. Since the beginning of nuclear medicine, radiolanthanides have been considered for use in radionuclide therapy. 153Sm, 149Pm, 161Tb, 166Ho, and 177Lu have been bound to monoclonal antibodies and peptides for treatment of various tumor types (1). 177Lu is a low-energy-electron–emitting radiolanthanide that is starting to be used as a replacement for the high-energy-electron–emitting nonlanthanide 90Y. Both 90Y-labeled and 177Lu-labeled somatostatin analogs and antibodies have shown promising results in the treatment of tumors. Promising preclinical results have been obtained with [177Lu-DOTA0,Tyr3]-octreotate (177Lu-DOTATATE) against various neuroendocrine tumors (1–3) and with 177Lu-labeled monoclonal antibodies against small peritoneal metastases of colorectal origin (4). In the latter study, using a murine model, the median survival time was statistically significantly longer after treatment with 177Lu-MN-14 than with 186Re-MN-14 or 90Y-MN-14 and was longer than with 131I-MN-14 although not statistically significantly so. Encouraging clinical results have also been achieved with 177Lu-DOTATATE and [177Lu-DOTA0-Tyr3]-octreotide (177Lu-DOTATOC) in the treatment of neuroendocrine tumors (5–9). Despite the encouraging early therapeutic results with 177Lu in radionuclide therapy, it is not known whether it is the optimal therapeutic radionuclide, and the investigation of other radionuclides for this purpose would thus be valuable. Because all lanthanides have similar chemical properties, they should have similar labeling procedures, and 177Lu might easily be replaced by other radiolanthanides. Furthermore, many other lanthanides also have large cross sections for neutron absorption, and as a result, high activities can often be produced.
As a first criterion for the therapeutic use of radiolanthanides, it is important to study their energy deposition in tumors and in normal tissue. The absorbed dose to normal tissue, and especially to critical organs, needs to be kept as low as possible. Thus, radionuclides with a low photon emission must be used. Photons are useful for diagnostic purposes and biokinetic studies, but normal tissues will be irradiated in an undesired way. Wessels and Rogus introduced a model for estimation of the influence of photons on the absorbed dose to normal tissue and a single large tumor for therapy with radiolabeled antibodies (10). Bernhardt et al. developed this model further to make it valid for different tumor sizes and biodistributions (11). They also stressed that this model can be useful even without information about the exact biokinetics (12). These models calculate the tumor–to–normal-tissue absorbed dose ratio (TND) and the tumor–to–normal-tissue mean absorbed dose rate ratio ( ). These models generate clear parameters for general dosimetric studies of the suitability of different radionuclides for therapy (11). In this study, only the
). These models generate clear parameters for general dosimetric studies of the suitability of different radionuclides for therapy (11). In this study, only the  is calculated because the biodistribution of the labeled substance needs to be known for calculating the TND.
is calculated because the biodistribution of the labeled substance needs to be known for calculating the TND.
Depending on the production route, either no-carrier-added (nca) or carrier-added (ca) radionuclides are obtained. High specific activity is necessary for systemic radionuclide therapy (1,13), especially when using peptides with pharmacologic side effects (14). All electron-emitting radiolanthanides investigated in this study can be produced with nuclear reactors (15), either directly or indirectly, or by using accelerators (1). A few radiolanthanides can be obtained from radionuclide generator systems (16).
The irradiation of stable lanthanide targets at nuclear reactors results in the neutron capture reaction (n,γ). For some targets, double neutron capture is required to produce the desired radioisotope, such as the 164Dy(n,γ)(n,γ)166Dy reaction. The radioactivity batch yield is, in principle, determined according to the equation: Eq. 1where h is the fraction of the isotope relevant for neutron capture, N is the atomic number of the lanthanide target, σ is the neutron capture cross section (most relevant is σth, the probability of absorbing thermal neutrons with energies of approximately 0.025 eV), Φ is the neutron flux, t is the irradiation time, and λ is the decay constant of the produced radioisotope (1). Neutron activation induced by epithermal neutrons at energies of between 1 eV and 1 keV may contribute to the radionuclide production yield. In contrast to the production routes discussed here, radiochemical separation of the radiolanthanide from the irradiated target is not possible and the radiolanthanide is ca. Nevertheless, because powerful nuclear reactors and isotopically enriched targets are available, this production route is among the more common, in particular if high thermal neutron fluxes of Φth ≥ 4·1014 n/cm2/s are available.
Eq. 1where h is the fraction of the isotope relevant for neutron capture, N is the atomic number of the lanthanide target, σ is the neutron capture cross section (most relevant is σth, the probability of absorbing thermal neutrons with energies of approximately 0.025 eV), Φ is the neutron flux, t is the irradiation time, and λ is the decay constant of the produced radioisotope (1). Neutron activation induced by epithermal neutrons at energies of between 1 eV and 1 keV may contribute to the radionuclide production yield. In contrast to the production routes discussed here, radiochemical separation of the radiolanthanide from the irradiated target is not possible and the radiolanthanide is ca. Nevertheless, because powerful nuclear reactors and isotopically enriched targets are available, this production route is among the more common, in particular if high thermal neutron fluxes of Φth ≥ 4·1014 n/cm2/s are available.
If the neutron capture reaction is followed by a β− decay of the primary produced nucleus, that is (n,γ) → β−, then chemical separation is possible, due to the new element formed, and the secondary radionuclide can be obtained nca.
Neutron irradiation of nuclei such as 233U induces fission (n, fission), thereby forming a spectrum of radiolanthanides with a maximum yield in the distribution of isotope mass at about 140. These light radiolanthanides may be chemically separated from the uranium target and from the other fission products. They are in nca form, but not necessarily isotopically pure, because several radioisotopes are produced for each lanthanide formed in the fission process.
Accelerators principally allow the formation of nca radiolanthanides, because the bombardment with accelerated protons, deuterons, and heavier ions results in nuclear processes such as (p,xn), (d,xn), (3He,xn), (α,xn), and many others, yielding products of different proton number. Individual radiolanthanides can be obtained according to the target chosen and the type and energy of the accelerated projectile, but absolute production yields may not reach those of reactor-based production routes.
High-energy proton irradiation results in fragmentation of the target nucleus (p, spallation). A spectrum of nca radionuclides is obtained. In addition to off-line radiochemical separation processes, online isotope separation facilities include a mass-separation facility. For the production of radiolanthanides, targets such as tantalum or tungsten are irradiated with protons of, usually, more than 600 MeV.
Several radiolanthanide generators are available, such as 134Ce/134La, 140Nd/140Pr, and 166Dy/166Ho, with the last being the most common. Key advantages of radionuclide generators include the availability of the daughter radionuclide in nca form and the convenience of obtaining the desired daughter radionuclide on demand, without having to rely on access to nuclear reactors or accelerators.
The aim of this work was to evaluate the dosimetric and production properties of electron- and positron-emitting radiolanthanides for radionuclide therapy. The investigated radiolanthanides were compared with the nonlanthanide radionuclides 90Y and 131I that are routinely used for clinical therapy today.
MATERIALS AND METHODS
Electron- and positron-emitting radiolanthanides with stable or long-lived daughter nuclides, half-lives between 1 h and 15 d, and low (<5) photon-to-electron (p/e) energy ratios (the sum of the total photon energy emitted per decay divided by the sum of the total electron energy emitted per decay) were selected, together with positron-emitting radiolanthanides proposed for systemic therapy (1,17,18). The electron-emitting radiolanthanides, together with their decay data, are given in Table 1, and the positron-emitting radiolanthanides are given in Table 2. The electron emitters 134Ce and 140Nd are listed in Table 2 instead of Table 1, because in practice they represent transient equilibria with their short-lived positron-emitting daughter nuclides 134La and 140Pr.
The  model was recently developed for general dosimetric evaluation of radionuclides (11,12) and is described briefly here. The
model was recently developed for general dosimetric evaluation of radionuclides (11,12) and is described briefly here. The  was calculated by a program written in MATLAB 6.5 (The MathWorks Inc.). The whole body was simulated as a 70-kg ellipsoid, with principal axes forming the ratio 1:1.8:9.27, and the tumors were represented by spheres of different sizes. Both the normal tissue and the tumors were assumed to be of unit density matter. The tumor sizes ranged from 1 cell, with a radius of 6.2 μm (1 ng), to a tumor with a radius of 29 mm (100 g). The activity distribution was assumed to be uniform, both in the ellipsoid and in the spheres. The mean absorbed dose rates to the tumor and whole body were calculated. The
was calculated by a program written in MATLAB 6.5 (The MathWorks Inc.). The whole body was simulated as a 70-kg ellipsoid, with principal axes forming the ratio 1:1.8:9.27, and the tumors were represented by spheres of different sizes. Both the normal tissue and the tumors were assumed to be of unit density matter. The tumor sizes ranged from 1 cell, with a radius of 6.2 μm (1 ng), to a tumor with a radius of 29 mm (100 g). The activity distribution was assumed to be uniform, both in the ellipsoid and in the spheres. The mean absorbed dose rates to the tumor and whole body were calculated. The  was calculated according to
was calculated according to Eq. 2where TNC is the tumor-to-normal-tissue activity concentration ratio (11), that is, the activity concentration in the tumor divided by the activity concentration in the normal tissue. Ee,i and Ep,i are the energies of the emitted electrons and photons, respectively, per transition i; ne,i and np,i are the numbers of emitted electrons and photons, respectively, per decay; ϕT,e,i, ϕT,p,i, ϕN,e,i, and ϕN,p,i are the absorbed fractions of electrons and photons in tumor and normal tissue, respectively; and mT and mN are the masses of the tumor and normal tissue, respectively. The absorbed fractions of electrons and positrons were calculated using Berger's point kernel data (19). The absorbed fractions for photons were taken from MIRD pamphlets 3 (20) and 8 (21). The decay data were taken from the Table of Radioactive Isotopes (22,23). The electrons emitted in the normal tissue were assumed to be locally absorbed, and thus ϕN,e,i was set at 1.
Eq. 2where TNC is the tumor-to-normal-tissue activity concentration ratio (11), that is, the activity concentration in the tumor divided by the activity concentration in the normal tissue. Ee,i and Ep,i are the energies of the emitted electrons and photons, respectively, per transition i; ne,i and np,i are the numbers of emitted electrons and photons, respectively, per decay; ϕT,e,i, ϕT,p,i, ϕN,e,i, and ϕN,p,i are the absorbed fractions of electrons and photons in tumor and normal tissue, respectively; and mT and mN are the masses of the tumor and normal tissue, respectively. The absorbed fractions of electrons and positrons were calculated using Berger's point kernel data (19). The absorbed fractions for photons were taken from MIRD pamphlets 3 (20) and 8 (21). The decay data were taken from the Table of Radioactive Isotopes (22,23). The electrons emitted in the normal tissue were assumed to be locally absorbed, and thus ϕN,e,i was set at 1.
The dependence of the TNC value on the  values was calculated for the radiolanthanide 177Lu.
values was calculated for the radiolanthanide 177Lu.  values were calculated for TNC values ranging from 2 to 100. The further calculations used a TNC value of 25; that is, the activity concentration was assumed to be 25 times higher in the tumor than in normal tissue.
values were calculated for TNC values ranging from 2 to 100. The further calculations used a TNC value of 25; that is, the activity concentration was assumed to be 25 times higher in the tumor than in normal tissue.
RESULTS
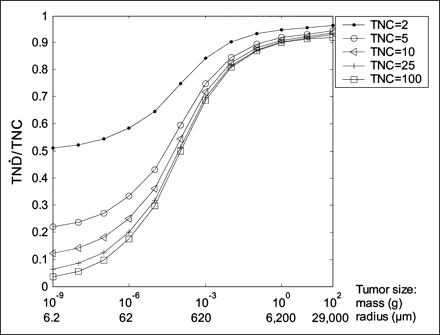
The dependence of the TNC value on the  values of 177Lu can be seen in Figure 1. The maximum possible
values of 177Lu can be seen in Figure 1. The maximum possible 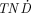 value is equal to the TNC value; therefore, the results are shown as the
value is equal to the TNC value; therefore, the results are shown as the  values divided by the TNC values. The shapes of the
values divided by the TNC values. The shapes of the 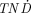 /TNC values as a function of tumor size will be similar for TNC values over 5. The
/TNC values as a function of tumor size will be similar for TNC values over 5. The  values divided by the TNC values differ mainly for small tumors (<10 μg).
values divided by the TNC values differ mainly for small tumors (<10 μg).
TN divided by TNC as function of tumor size for electron-emitting radiolanthanide 177Lu.
divided by TNC as function of tumor size for electron-emitting radiolanthanide 177Lu.
The  values as a function of tumor size for the electron-emitting radiolanthanides studied are shown in Figure 2. The
values as a function of tumor size for the electron-emitting radiolanthanides studied are shown in Figure 2. The 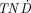 values increase with tumor size for all radionuclides because of the increase of the specific absorbed fraction in the tumor. Energy absorbed in the normal tissue will not be affected to the same extent when the size of the tumor is increased. Compared with 90Y and 131I—the reference nuclides used—many radiolanthanides had higher
values increase with tumor size for all radionuclides because of the increase of the specific absorbed fraction in the tumor. Energy absorbed in the normal tissue will not be affected to the same extent when the size of the tumor is increased. Compared with 90Y and 131I—the reference nuclides used—many radiolanthanides had higher  values for both smaller and larger tumors. The radiolanthanides that showed the highest
values for both smaller and larger tumors. The radiolanthanides that showed the highest  values were 153Sm, 161Tb, 169Er, 175Yb, and 177Lu. 169Er displayed higher
values were 153Sm, 161Tb, 169Er, 175Yb, and 177Lu. 169Er displayed higher  values than did 177Lu for all tumor sizes studied and the highest
values than did 177Lu for all tumor sizes studied and the highest  values of all radiolanthanides studied for tumors larger than 10 μg. The
values of all radiolanthanides studied for tumors larger than 10 μg. The 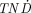 values as a function of tumor size were similar for 177Lu, 175Yb, and 161Tb, with somewhat higher
values as a function of tumor size were similar for 177Lu, 175Yb, and 161Tb, with somewhat higher  values for the latter for tumors smaller than 100 μg. For very small tumors (<10 μg), 161Ho displayed the highest
values for the latter for tumors smaller than 100 μg. For very small tumors (<10 μg), 161Ho displayed the highest  values of all electron-emitting radiolanthanides studied, whereas for tumors larger than 1 mg, 161Ho had rather low
values of all electron-emitting radiolanthanides studied, whereas for tumors larger than 1 mg, 161Ho had rather low  values.
values.
TN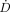 as function of tumor size for electron-emitting radiolanthanides. In all panels, 177Lu, 90Y, and 131I are shown for comparison.
as function of tumor size for electron-emitting radiolanthanides. In all panels, 177Lu, 90Y, and 131I are shown for comparison.
142Pr, 145Pr, and 166Ho had 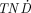 values similar to those of 90Y. The
values similar to those of 90Y. The  values of 143Pr, 149Pm, 150Eu, 159Gd, 165Dy, 176mLu, and 179Lu were higher than those of 90Y for all tumor sizes studied.
values of 143Pr, 149Pm, 150Eu, 159Gd, 165Dy, 176mLu, and 179Lu were higher than those of 90Y for all tumor sizes studied.
The electron-emitting radiolanthanides with the highest p/e ratios (>1.8), namely 140La, 142La, 150Pm, 156Eu, and 157Eu, emit medium- and high-energy electrons (>350 keV), leading to low  values for all tumor sizes studied. 172Tm and 173Tm have electron energies similar to those of 140La, 156Eu, and 157Eu, but the 2 radionuclides of thulium have higher
values for all tumor sizes studied. 172Tm and 173Tm have electron energies similar to those of 140La, 156Eu, and 157Eu, but the 2 radionuclides of thulium have higher  values than the 3 other radiolanthanides mentioned, because of their lower p/e ratios (0.9 and 1.3, respectively). 167Ho and 167Tm have high p/e ratios (>1.2) and low electron energies (<200 keV), and the
values than the 3 other radiolanthanides mentioned, because of their lower p/e ratios (0.9 and 1.3, respectively). 167Ho and 167Tm have high p/e ratios (>1.2) and low electron energies (<200 keV), and the  values for larger tumors are low.
values for larger tumors are low.
The  values as a function of tumor size for the positron-emitting radiolanthanides studied are presented in Figure 3. In general, all positron emitters had low
values as a function of tumor size for the positron-emitting radiolanthanides studied are presented in Figure 3. In general, all positron emitters had low 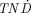 values, although 141Nd had relatively high
values, although 141Nd had relatively high 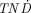 values for small tumors.
values for small tumors.
TN as function of tumor size for positron-emitting radiolanthanides. Corresponding values for 90Y and 131I are shown for comparison.
as function of tumor size for positron-emitting radiolanthanides. Corresponding values for 90Y and 131I are shown for comparison.
Routine clinical application requires the availability of radionuclides both at sufficient radioactivities and at high specific activities. 177Lu can be produced in ca form by direct neutron activation of 176Lu, with a high cross section (∼2,100 b), or in nca form via indirect production from β-decay of reactor-produced 177Yb (24,25). In theory, it should be possible to produce 177Lu with a specific activity of around 4.1 TBq/mg, according to Eq. 3where λ is the decay constant and N is the number of atoms in 1 mg.
Eq. 3where λ is the decay constant and N is the number of atoms in 1 mg.
153Sm can be produced by neutron bombardment of 152Sm at a rather high specific activity of up to 144 GBq/mg (26–28), because of the high cross section (206 b) of the thermal neutron capture reaction. Because this is an (n,γ) reaction, 153Sm will be obtained in the ca form.
161Tb can be produced after neutron capture of 160Gd according to 160Gd(n,γ) 161Gd (half-life, 3.6 min) → β−→ 161Tb (29). 161Tb subsequently needs to be chemically separated from the neighboring gadolinium (29). Via this route, 161Tb is in principle obtained in an nca form with a theoretic specific activity of 4.3 GBq/mg, according to Equation 3. No direct route is available, and double neutron capture by 159Tb would be the only alternative, providing a rather low specific activity.
169Er was the radiolanthanide with the highest 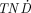 values for most of the tumors sizes studied. 169Er can be produced through neutron capture of stable 168Er by the reaction 168Er(n,γ)169Er. However, as the neutron capture cross section of this process is rather low (2 b), the specific activity of the 169Er produced will be low and the nuclide will be obtained in the ca form.
values for most of the tumors sizes studied. 169Er can be produced through neutron capture of stable 168Er by the reaction 168Er(n,γ)169Er. However, as the neutron capture cross section of this process is rather low (2 b), the specific activity of the 169Er produced will be low and the nuclide will be obtained in the ca form.
175Yb can be produced by thermal neutron bombardment of ytterbium targets isotopically enriched with 174Yb, that is, by the reaction 174Yb(n,γ)175Yb. In contrast to 169Er, the specific activities of 175Yb are high because of the neutron capture cross section of this process (100 b), but 175Yb will still be obtained in a ca form.
161Ho is available at particle accelerators, including medical-type cyclotrons. One route is the α-irradiation of 159Tb, or the 159Tb(α,2n)161Ho route. The other route is the irradiation of dysprosium targets using protons or deuterons: 161Dy(p,n), 162Dy(d,2n), or 160Dy(d,n), for example. In theory, 161Ho can be produced with a specific activity of 290 TBq/mg, according to Equation 3. After radiochemical separation, 161Ho is obtained nca.
The only radiolanthanide of 143Pr, 149Pm, 150Eu, 159Gd, 165Dy, 176mLu, and 179Lu that can be produced at high specific activities and nca is 149Pm. 149Pm can be produced by the indirect route 148Nd(n,γ)149Nd → 149Pm (30). With this method, it would be possible to obtain theoretic specific activities of 15 GBq/mg, according to Equation 3.
142Pr and 165Dy are available in ca form only, and production rates of nca 145Pr may be too low for routine (and commercial) applications. Thus, nca 166Ho available from a 166Dy/166Ho generator, rather than via the 165Ho(n,γ)166Ho process, might be an option for dedicated applications in radionuclide therapy. With the generator, a theoretic specific activity of 26 TBq/mg can be achieved, according to Equation 3.
DISCUSSION
The  model is an analytic method that is easy to use. In this study, a constant TNC and a uniform activity distribution in both the tumor and normal tissue were assumed. The
model is an analytic method that is easy to use. In this study, a constant TNC and a uniform activity distribution in both the tumor and normal tissue were assumed. The  model is a convenient method for estimating the ratio between the absorbed dose rates to tumors of different sizes and to normal tissue. In this study, the normal tissue was assumed to be the whole body. The bone marrow is usually one of the dose-limiting organs in radionuclide therapy. Absorbed dose to the bone marrow is difficult to estimate, but bone marrow toxicity correlates, in some applications, with absorbed dose to the whole body (31). Whether the same is true for other clinical situations remains to be studied.
model is a convenient method for estimating the ratio between the absorbed dose rates to tumors of different sizes and to normal tissue. In this study, the normal tissue was assumed to be the whole body. The bone marrow is usually one of the dose-limiting organs in radionuclide therapy. Absorbed dose to the bone marrow is difficult to estimate, but bone marrow toxicity correlates, in some applications, with absorbed dose to the whole body (31). Whether the same is true for other clinical situations remains to be studied.
It is possible to determine the ratio between the absorbed dose to the tumor and normal tissue, TND, by adding biokinetic data in the form of changes in the TNC and taking the half-lives of the radionuclides into consideration. Because the TNC varies considerably between different substances and tissues and also with time, such calculations are needed for a detailed evaluation of the usefulness of interesting radionuclides bound to a specific radiopharmaceutical. We chose to perform the calculations for a constant TNC value of 25. This choice was based on the assumption that a large tumor would need an absorbed dose of 100 Gy to be sterilized, whereas normal tissue, such as bone marrow, could endure an absorbed dose of 4 Gy. However, Figure 1 shows that the shape of the 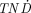 /TNC values as a function of tumor size will not vary significantly for TNC values higher than about 5. Therefore, using another TNC value will yield results and conclusions similar to those obtained in this study. The results are not prejudiced by any biokinetics, and they can be used as a guide for further studies on a defined pharmaceutical and tumor model. Especially, our results demonstrate radiolanthanides not suitable for therapy of patients because of the high contribution of photons, irrespective of the radiopharmaceutical used.
/TNC values as a function of tumor size will not vary significantly for TNC values higher than about 5. Therefore, using another TNC value will yield results and conclusions similar to those obtained in this study. The results are not prejudiced by any biokinetics, and they can be used as a guide for further studies on a defined pharmaceutical and tumor model. Especially, our results demonstrate radiolanthanides not suitable for therapy of patients because of the high contribution of photons, irrespective of the radiopharmaceutical used.
A large abundance of photons will reduce the therapeutic potential of the radionuclide, as is well demonstrated by the  model, which shows decreasing
model, which shows decreasing 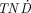 values with increasing photon emission. The model also gives information about the tumor sizes for which the absorbed fraction of emitted electrons is high. On the basis of the results of the
values with increasing photon emission. The model also gives information about the tumor sizes for which the absorbed fraction of emitted electrons is high. On the basis of the results of the  calculations, the radiolanthanides can be divided into 5 different groups. To the first group belong the low-energy (100−250 keV) electron emitters with low photon abundance (p/e < 0.3) and high
calculations, the radiolanthanides can be divided into 5 different groups. To the first group belong the low-energy (100−250 keV) electron emitters with low photon abundance (p/e < 0.3) and high  values even for rather small tumors: 153Sm, 161Tb, 169Er, 175Yb, and 177Lu. In this group, the only radionuclides that can be produced in sufficient amounts and nca are 177Lu and 161Tb. The use of radionuclides in nca form and with high specific activities is important when biologically active peptides such as somatostatin analogs are used. In other cases, the use of radionuclides with lower specific activities and in ca form might be possible. 177Lu and 161Tb have similar
values even for rather small tumors: 153Sm, 161Tb, 169Er, 175Yb, and 177Lu. In this group, the only radionuclides that can be produced in sufficient amounts and nca are 177Lu and 161Tb. The use of radionuclides in nca form and with high specific activities is important when biologically active peptides such as somatostatin analogs are used. In other cases, the use of radionuclides with lower specific activities and in ca form might be possible. 177Lu and 161Tb have similar  values and physical half-lives. However, the different emission of Auger electrons will make these 2 radionuclides of special interest in studying the Auger effect for various internalizing radiopharmaceuticals, such as radiolabeled somatostatin analogs (32).
values and physical half-lives. However, the different emission of Auger electrons will make these 2 radionuclides of special interest in studying the Auger effect for various internalizing radiopharmaceuticals, such as radiolabeled somatostatin analogs (32).
The lower specific activities of 153Sm, 169Er, and 175Yb are quite acceptable for specific therapeutic strategies such as radiosynoviorthesis or palliative treatment of disseminated bone metastases. Additionally, significant batch activities are easily achievable for 153Sm and 175Yb. However, their use for the labeling of tumor receptor– or antigen-targeting vectors might be limited because of the much higher specific activities required.
The second group consists of the Auger-electron emitters 161Ho and 167Tm. These 2 radiolanthanides emit photons in high abundance (p/e > 1.2). 161Ho displays high  values for small tumors because of its low electron energies (Table 1) but has low
values for small tumors because of its low electron energies (Table 1) but has low  values for tumors larger than 1 mg (radius, 0.62 mm), because of the significant accompanying photon emission. Also, the low-energy electron emitter 161Tb might be included in this group because of the significant quantity of Auger-electrons emitted. These radiolanthanides emit low-energy β- or conversion electrons that make them especially interesting for radiopharmaceuticals that are internalized by the tumor cells but not by the dose-limiting normal cells. If these nuclides are internalized into the nucleus of the cell, the influence on the absorbed dose to the nucleus from the low-energy electrons will be increased, compared with the absorbed dose from the electrons with higher energies and the photons. In other words, when one is calculating the absorbed dose rate to the cell nucleus, the
values for tumors larger than 1 mg (radius, 0.62 mm), because of the significant accompanying photon emission. Also, the low-energy electron emitter 161Tb might be included in this group because of the significant quantity of Auger-electrons emitted. These radiolanthanides emit low-energy β- or conversion electrons that make them especially interesting for radiopharmaceuticals that are internalized by the tumor cells but not by the dose-limiting normal cells. If these nuclides are internalized into the nucleus of the cell, the influence on the absorbed dose to the nucleus from the low-energy electrons will be increased, compared with the absorbed dose from the electrons with higher energies and the photons. In other words, when one is calculating the absorbed dose rate to the cell nucleus, the  values will increase if the radionuclide is internalized into the tumor cells but not into the normal cells. The short range of Auger electrons might require that labeled compounds approach the cell nucleus subsequent to internalization, in turn requiring new and efficient chemical and radiopharmaceutical targeting strategies. Another problem with low-energy electrons is that all cells must be targeted in order to achieve a uniform distribution of absorbed dose in the tumor. It has been demonstrated theoretically that therapy with radionuclides emitting very-low-energy electrons would be difficult if not all the cells are targeted (33,34). 161Ho, 167Tm, and 161Tb might be useful radionuclides for further investigations of the significance of Auger electrons in therapeutic applications. Another radiolanthanide that also demonstrates a relatively high emission of Auger electrons is 153Sm. However, 153Sm can be produced only ca and in too low a specific activity.
values will increase if the radionuclide is internalized into the tumor cells but not into the normal cells. The short range of Auger electrons might require that labeled compounds approach the cell nucleus subsequent to internalization, in turn requiring new and efficient chemical and radiopharmaceutical targeting strategies. Another problem with low-energy electrons is that all cells must be targeted in order to achieve a uniform distribution of absorbed dose in the tumor. It has been demonstrated theoretically that therapy with radionuclides emitting very-low-energy electrons would be difficult if not all the cells are targeted (33,34). 161Ho, 167Tm, and 161Tb might be useful radionuclides for further investigations of the significance of Auger electrons in therapeutic applications. Another radiolanthanide that also demonstrates a relatively high emission of Auger electrons is 153Sm. However, 153Sm can be produced only ca and in too low a specific activity.
The third group consists of the medium-energy (280–470 keV) electron emitters with low photon abundance (p/e < 0.2): 143Pr, 149Pm, 150Eu, 159Gd, 165Dy, 176mLu, and 179Lu. These radiolanthanides have 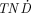 values similar to that of 131I for small tumors and even higher
values similar to that of 131I for small tumors and even higher  values for larger tumors, because of their lower photon emission. However, the only radionuclide that can be produced in high specific activities (15 GBq/mg) and nca is 149Pm.
values for larger tumors, because of their lower photon emission. However, the only radionuclide that can be produced in high specific activities (15 GBq/mg) and nca is 149Pm.
The fourth group comprises the high-energy (>650 keV) electron emitters with low photon abundance (p/e < 0.1). These are 142Pr, 145Pr, and 166Ho, of which 166Ho is the only nuclide that can be produced in sufficient amounts. High-energy-electron–emitting radionuclides are suitable for large tumors (>1 g), but the low absorbed electron energy fraction in small tumors might limit their use for treatment of disseminated diseases with small tumor nests (34,35). However, nonuniform activity distributions in tumors might necessitate the use of high-energy electron emitters to obtain a uniform absorbed dose (34). A greater number of experimental studies are required, and the generator-produced high-energy-electron–emitting 166Ho might be a valuable component of well-designed experimental studies.
The fifth group contains radiolanthanides with a high photon emission (p/e > 0.9).These are 140La, 142La, 150Pm, 156Eu, 157Eu, 167Ho, 172Tm, 173Tm, and the positron-emitting radiolanthanides. Their p/e values result in low  values for all tumor sizes. Using these radionuclides for therapy will result in a high absorbed dose to the whole body and most probably in bone marrow toxicity. All these radionuclides have higher p/e ratios or higher mean electron energy and lower
values for all tumor sizes. Using these radionuclides for therapy will result in a high absorbed dose to the whole body and most probably in bone marrow toxicity. All these radionuclides have higher p/e ratios or higher mean electron energy and lower  values than does 131I (p/e = 2, E = 182 keV). Therefore, in searches for more optimal radionuclides, one criterion should be a p/e ratio lower than that of 131I (12).
values than does 131I (p/e = 2, E = 182 keV). Therefore, in searches for more optimal radionuclides, one criterion should be a p/e ratio lower than that of 131I (12).
In this study, we identified a total of 6 radiolanthanides that might be suitable for binding to tumor-seeking pharmaceuticals, namely 149Pm, 161Tb, 161Ho, 166Ho, 167Tm, and 177Lu. Five of these radionuclides have half-lives of between 1.1 and 9.2 d, whereas 161Ho has a half-life of only 2.5 h. This short half-life might limit the use of this radionuclide for systemic therapies, but it might be suitable for local therapies, such as intratumoral therapy of brain tumors. The 6 selected radiolanthanides include electron emitters within the low-energy (E < 250 keV), medium-energy (280−470 keV), and high-energy (>650 keV) regions.
CONCLUSION
Six radiolanthanides that might be suitable for radionuclide therapy were selected, among them the low-energy electron emitters 177Lu, 161Tb, 167Tm, and 161Ho. Because production routes are available to obtain 161Tb and 177Lu routinely in nca form, these radionuclides might be interesting choices for dedicated radionuclide therapeutic applications. 161Ho, with its short half-life, might be suitable for local radionuclide therapy. If radionuclides emitting higher electron energies are needed, the medium-energy electron emitter 149Pm and the high-energy electron emitter 166Ho may, in principle, be used instead of 90Y.
Acknowledgments
This study was supported by the Swedish Cancer Society (grants 3427 and 4956) and the King Gustav V Jubilee Clinic Foundation, Sweden, and was carried out within the European Cooperation in the Field of Science and Technology programs COST D18 and COST B12.
References
- Received for publication October 11, 2005.
- Accepted for publication January 11, 2006.