Abstract
Changes in regional myocardial perfusion throughout the entire coronary vascular tree, as opposed to changes in the worst regional perfusion defect, have not been described during long-term regression or progression of coronary artery disease (CAD) or related to clinical outcomes. Methods: Four-hundred nine patients with CAD undergoing dipyridamole PET at baseline and after 2.6 ± 1.4 y were followed over 5 more years for coronary events. PET images were objectively quantified by automated software for changes in severity of the (i) baseline worst quadrant, indicating the worst flow-limiting stenosis at baseline PET; (ii) follow-up worst quadrant, indicating the worst stenosis on follow-up PET; and (iii) maximal change quadrant, indicating the largest change of any same quadrant pair from baseline–to–follow-up images. Results: At follow-up PET, new regional perfusion defects were seen in 40% of patients. In 77% of patients, the greatest change was in a quadrant different from the worst baseline defect. The maximal change quadrant improved in 70% of patients on intense lifestyle and pharmacologic lipid treatment, in 48% on moderate treatment, and in 39% on poor treatment (P < 0.0001). Combined quadrant changes integrated throughout the heart independently predicted cardiovascular events at long-term follow-up. In contrast, changes of any single baseline–to–follow-up quadrant pair did not. Conclusion: By PET, 77% of patients with CAD had the greatest perfusion changes in areas different from the baseline worst perfusion defect and 40% had new perfusion defects. Changes in perfusion defects throughout the entire coronary vascular tree predicted coronary events, whereas changes in the worst flow-limiting stenosis at baseline or in any one segment of myocardium did not. To our knowledge, these data provide the first direct evidence on mechanisms for disproportionately greater reduction in cardiac events than changes in single stenosis severity with lipid treatment.
Coronary atherosclerosis progresses by 2 mechanisms—gradual progressive lipid accumulation causing coronary artery stenosis or sudden plaque rupture associated with localized inflammation and thrombosis that partially or completely blocks the artery. Gradual accumulation of lipid with progressive narrowing is greatest at the downstream end or exit of the stenosis in the region of low shear (1,2). Furthermore, a small amount of lipid accumulating within a moderate stenosis causes more severe additional narrowing than a comparable amount of lipid accumulation in nonstenotic arterial segments. Therefore, progression due to gradual lipid accretion is characterized by existing stenosis becoming more severe. In contrast, plaque rupture commonly causes new lesions at sites of no significant prior stenosis that may be silent or associated with sudden cardiac events or unstable coronary syndromes (3–9). A spectrum of both types of progression may occur in different proportions in the same or different coronary arteries of any individual.
Similarly, regression of coronary atherosclerosis is characterized by modestly decreased stenosis severity and absence of new lesions in association with reduced cardiac events proportionately greater than the modest reduction in stenosis severity (3,4,10–13). The essential concept is that worsening of a flow-limiting stenosis over time most likely reflects the mechanism of gradual accumulation of lipids, whereas the appearance of new flow-limiting stenosis not present at baseline most likely reflects the mechanism of plaque rupture. Because active, progressive coronary atherosclerosis is multicentric (14–17), assessing changes in the entire coronary artery tree is essential for identifying and quantifying progression or regression of coronary artery disease (CAD), its response to risk factor treatment, and for predicting risk of clinical events.
We have previously reported that combined intense lifestyle and pharmacologic lipid treatment to specific risk factor goals improves myocardial perfusion and markedly reduces coronary events, more than either lifestyle or pharmacologic treatment alone over 5-y follow-up (18). However, despite the severity of the baseline perfusion defect being a good predictor of clinical events, the changes in the most severe perfusion defect from baseline–to–follow-up PET over 2.6 ± 1.4 y were poorly predictive of coronary events over the next 5 y, a surprising finding.
Therefore, to explain this unexpected finding based on the literature above, we hypothesized that changes in myocardial perfusion throughout the entire coronary vascular tree in regions other than the baseline worst perfusion abnormalities by sequential dipyridamole PET perfusion imaging would (i) predict clinical outcomes at long-term follow-up better than the changes of the most severe perfusion defect due to the single worst flow-limiting coronary stenosis at baseline, (ii) correlate with intensity of risk factor treatment, (iii) identify new flow-limiting stenosis in new regions without a significant perfusion defect at baseline, and (iv) demonstrate worsening or improvement of the worst baseline flow-limiting stenosis consistent with a mechanism of gradual lipid accumulation or removal depending on risk factor treatment.
In principle, precise myocardial perfusion mapping should noninvasively provide objective quantification of the net balance of mixed progression or regression of diffuse disease and localized stenosis for each coronary artery and for the entire heart that is not possible with coronary arteriography or intracoronary ultrasounds. For testing these new hypotheses, we developed automated software for objectively quantifying PET perfusion defects for changes in the same region as the most severe perfusion defect at baseline or changes in other baseline–to–follow-up paired regions that are not the same as the region with the most severe defect at baseline.
MATERIALS AND METHODS
Study Patients
A total of 409 consecutive, unselected patients with stable CAD underwent rest-dipyridamole myocardial perfusion imaging by PET at baseline and at follow-up averaging 2.6 y later and were followed for 5 more years for cardiovascular events. All patients signed an informed consent approved by the University of Texas Committee for Protection of Human Subjects. Having 2 sequential cardiac PET studies with abnormal stress perfusion images at baseline was the criterion for inclusion in the study. The abnormal dipyridamole PET was a prerequisite for the study but not a coronary arteriogram. The follow-up PET scan and its timing were determined by several factors, including routine follow-up in an intensive treatment program, referral for follow-up by the patient's physician, direct request by the patient for a follow-up PET, or interim clinical problems.
At the follow-up PET study, patients were categorized by blinded observers using predefined objective criteria into 3 groups based on the intensity of medical therapy during the interval between the 2 PET studies. As previously described in detail (18), patients in the poor treatment group were not on a diet or a lipid active medication or were actively smoking. Patients in the moderate therapy group were instructed in an American Heart Association diet and took lipid active drugs not dosed to specific goals or adhered to a very low fat diet with ≤10% of calories as fat without lipid-lowering drugs. Patients in the maximal treatment group adhered to a strict diet (<10% fat calories), regular exercise, and lipid active medications dosed to target low-density lipoproteins (LDLs) <90 mg/dL, high-density lipoproteins (HDLs) >40 mg/dL, and triglycerides <100 mg/dL.
Patients with only 12 mo or less between baseline and follow-up PET, defined as inadequate for predicting long-term events, were analyzed as a separate group for PET endpoints. Accordingly, the minimum treatment period was 1 y. Patients with percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG) in the interval between the 2 PET studies were considered separately as an internal comparison because the intervention would alter myocardial perfusion separately from risk factor treatment. Follow-up for clinical outcome was obtained at a mean interval of 5 y after the final PET study for death, nonfatal myocardial infarction, PCI, CABG, or stroke.
We did not exclude patients with resting defects for several reasons: (i) such selection might introduce a possible bias; (ii) even if the defect were a fixed scar, the changes in the rest of the heart provide important data on progression–regression in borderzone regions or new stenosis in other myocardial regions; and (iii) the patients with no improvement due to a fixed scar despite optimal, intense medical therapy would reduce the changes we observed and be against our conclusions that remained significant despite their inclusion. Therefore, including patients with resting defects is the most conservative statistically correct approach but patients with significant defects on the resting perfusion scan were also analyzed separately. Patients with severe liver or renal dysfunction or with coronary revascularization within 6 mo from the baseline PET study were excluded.
PET
PET was performed using the University of Texas–designed, Posicam, bismuth germanate multislice tomograph with a reconstructed resolution of 10-mm full width at half maximum as previously described (18–24). Transmission images to correct for photon attenuation contained 100–150 million counts. Emission images obtained after intravenous injection of 666 MBq (18 mCi) of cyclotron-produced 13N contained 20–40 million counts.
At 40 min after administration of the first dose of ammonia, dipyridamole (0.142 mg/kg/min) was infused for 4 min. Four minutes after infusion was complete, a second dose of 666 MBq (18 mCi) of 13N-ammonia was injected intravenously. Four minutes later to allow blood-pool clearing, PET was repeated by the same protocol as for the resting study. For angina, aminophylline (125 mg) was given intravenously. The follow-up PET scan was obtained at a mean of 2.6 y after the baseline PET.
Automated Quantitative Analysis of PET Images
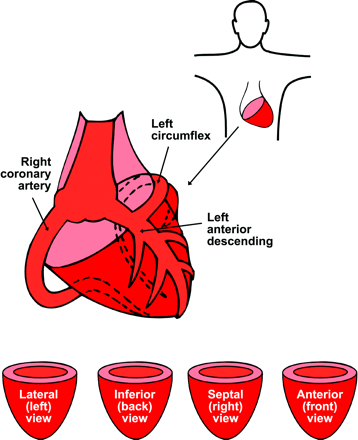
Completely automated analysis of severity–size of PET abnormalities was performed by previously described software (18,21,23,24). A 3-dimensional (3D) restructuring algorithm generates true short- and long-axis views from PET transaxial cardiac images, perpendicular to and parallel to the long axis of the left ventricle (LV). Circumferential profiles are used to reconstruct 3D topographic views of the LV showing relative regional activity distribution divided into lateral, inferior, septal, and anterior quadrant views of the 3D topographic display in Figure 1.
Orientation of PET perfusion images.
Mean activity in each quadrant is normalized to the maximum 2% of pixels in the whole heart dataset, regions of each quadrant are identified having values outside 97.5% confidence intervals (CI) or 2.5 SD outside normal values of 20 healthy control subjects without risk factors or family history of CAD (average age, 52 ± 9 y old). The percentage of circumferential profile units outside the 97.5% CI was calculated automatically in software. In addition, 10 patients underwent repeated sequential dipyridamole PET on different days to establish reproducibility of the quantitative measures of the size and severity of perfusion defects.
PET Definitions
Severity of a perfusion defect is quantified as the lowest quadrant average relative activity—that is, the average relative activity for the quadrant having the lowest average activity of anterior, septal, lateral, and inferior quadrants for each subject, expressed as the percentage of the highest 2% of activity in the image dataset. Size of perfusion defects is quantified as the percentage of the whole cardiac image outside the 97.5% CI or 2.5 SD of healthy controls. Combined size and severity of perfusion defects is defined as the percentage of the whole cardiac image with relative activity of <60% of maximum activity (100%) that is 3.0 SD below the mean maximum activity of healthy controls. At follow-up study, a change in activity within ± 2.5 SD of our PET reproducibility data is considered not significant. Table 1 lists measurement values for the 20 healthy subjects and for the 10 patients with serial PET for reproducibility. Changes greater than this ± 2.5 SD of repeated scans were considered significantly changed as better or worse.
Normal Values and Reproducibility
PET Endpoints
For each PET scan, change in severity is measured objectively by automated software as the change from baseline–to–follow-up dipyridamole PET of the quadrant average activity in relative normalized activity units for the quadrant pairings of baseline and follow-up images listed below. The entire group of patients was analyzed separately for the change in each of the following categories and then for mixed changes with worsening in one part of the heart and improvement in another part of the heart.
Baseline Worst Quadrant (BW).
BW is the quadrant with the minimum average quadrant activity after dipyridamole on the baseline PET, indicating the quadrant with the most severe defect representing the most severe flow-limiting stenosis at baseline. For changes at follow-up PET, this BW is compared with the same quadrant on follow-up dipyridamole PET images.
Follow-up Worst Quadrant (FuW).
FuW is the quadrant with the minimum average activity on the follow-up dipyridamole PET. FuW may be different from or the same as the BW.
Maximal Change Quadrant (MCh).
MCh is any quadrant with the greatest change from baseline to follow-up regardless of the presence or severity of baseline perfusion defects.
Combined Quadrants Change.
Combined Quadrants change is based on objectively measured changes in the BW, the FuW, and the MCh altogether. The Combined Quadrants change was defined as disease progression if all of these 3 baseline–to–follow-up comparisons—BW, FuW, and MCh—showed worsening. The Combined Quadrants change was defined as disease regression if all 3 baseline–to–follow-up comparisons showed improvement. The Combined Quadrants change was defined as mixed if neither progression nor regression criteria were met (specifically, if the change in the BW was opposite to the change in the FuW or MCh unless the FuW was the same quadrant as the BW; in that case, improvement or worsening in the MCh determined the final categorization as regression or progression, respectively). These quadrant pairings are summarized in Table 2.
Quadrant Pairings
Figure 2 illustrates changes from baseline–to–follow-up dipyridamole stress images. The red color signifies normal maximum perfusion after dipyridamole. The small orange area signifies a mild small dipyridamole-induced perfusion defect. The green medium-sized area signifies a moderate medium-sized dipyridamole-induced perfusion defect. The large blue area signifies a severe large dipyridamole-induced defect. This color scale is the same as for subsequent PET scans. The severity and size of perfusion defects and the different patterns of change from baseline–to–follow-up PET as objectively measured by automated software are illustrated by the following comparisons in Figure 2: BW at follow-up may be worse (arrow A) or better (arrow B). FuW may be the same quadrant as baseline (arrow A) or a different quadrant than at baseline (arrow C). The MCh may be a different quadrant than the baseline worst, either worse (arrow C) or better (arrow D).
Schematic of stress PET images after dipyridamole at baseline compared with follow-up PET in various quadrant pairings. Small orange area signifies a mild small dipyridamole-induced perfusion defect. Green medium-sized area signifies a moderate medium-sized dipyridamole-induced perfusion defect. Large blue area signifies a severe large dipyridamole-induced defect. BW at follow-up may be worse (arrow A) or better (arrow B). FuW may be the same quadrant as baseline (arrow A) or a different quadrant than at baseline (arrow C). MCh may be a different quadrant than the baseline worst, either worse (arrow C) or better (arrow D).
Statistical Analysis
Categoric or binary discrete variables are reported as frequencies and percentages with significant differences among groups determined by χ2 testing. Continuous variables are reported as mean ± 1 SD with significance of differences among groups determined by ANOVA with Bonferroni–Dunn post hoc correction. Correlations were calculated using a Spearman rank correlation coefficient. Statistica for Windows 5.1 (Statsoft Inc.) was used for all statistical calculations.
Stepwise multivariate logistic regression analyses adjusted for severity of the baseline PET defects were performed on all treated patients with the dependent variable being cardiovascular events—that is, cardiac death, nonfatal myocardial infarction, PCI, CABG, or stroke, over 5-y follow-up. The independent variables were as follows: (i) 15 clinical variables involving risk factors and intensity of treatment; (ii) 5 continuous values of the changes in severity on dipyridamole perfusion images at baseline and follow-up of the above 3 categories of quadrant pairs; (iii) change in size; (iv) change in combined size–severity of the perfusion defects; and (v) the discrete Combined Quadrants change of progression, regression, or mixed as defined above.
RESULTS
Baseline and follow-up PET scans were obtained in 409 patients with an average of 2.6 ± 1.4 y between scans. Baseline demographics of the patient population are in Table 3. Follow-up for cardiac events was obtained on all patients over 5 y after the final PET scan. Figure 3A illustrates progression with a new more severe defect (inferior) at follow-up PET in a different quadrant than at baseline (lateral). Figure 3B illustrates regression on PET perfusion images.
(A) PET perfusion images at rest (top row) and after dipyridamole (middle row) at baseline and after dipyridamole at follow-up (bottom row) illustrating progression. In color bar, white indicates the highest myocardial uptake of radionuclide reflecting the highest myocardial perfusion, with red being the next highest and progressively lower perfusion indicated by color gradations from red to yellow, green, and blue. At baseline, the lateral quadrant has the worst defect, whereas at follow-up, the inferior wall has the worst defect, illustrating that most patients had changes in quadrants other than the baseline worst quadrant. (B) PET perfusion images after dipyridamole at baseline and at follow-up illustrating regression.
Patient Characteristics
The MCh pair, for either better or worse, was different from the BW in 316 of 409 or 77% of the patients; MCh coincided with BW in only 93 of 409 or 23% of patients. This observation indicates that for most patients the greatest perfusion changes were seen in regions other than the BW.
To avoid any potential bias due to patients with resting myocardial scar, all 409 patients were divided into those with and those without severe resting perfusion defects. A severe resting perfusion defect indicating myocardial scar was defined as >15% of the LV outside ± 2 SD of normal on the resting image. Of the 409 patients, 99 or 24% had a perfusion defect on the resting image that was outside ± 2 SD of healthy subjects. For these patients with a myocardial scar, the MCh, for either better or worse, was different from the BW in 75% of patients with myocardial scar as defined above. For the remaining 310 patients of the 409 with <15% of the LV outside ± 2 SD of normal on the resting image, the MCh was different from the BW in 78% of patients without myocardial scar, comparable to the patients with myocardial scar. The results are the same if myocardial scar at baseline was defined as >15% of the LV with <60% of maximum activity. Therefore, the presence of a baseline myocardial scar did not bias our results.
To examine these changes in a different way, we also compared the location of the FuW with the location of the BW. FuW was different from BW in 162 of 409 or 40% patients and coincided with BW in 247 of 409 or 60%. When FuW was different from BW, it had a more severe defect than the BW in 84 or 21% of patients. This observation illustrates that disease progression on follow-up PET frequently involved a quadrant different from baseline and, for 21% of patients, the new defects were worse than the worst defect at baseline.
Of the 409 patients, 45 had a revascularization procedure in the interval between the first and second study and 38 had a follow-up PET scan within 12 mo from baseline. The remaining 326 patients were assigned to 1 of the 3 medical treatment groups with comparable baseline clinical characteristics (18).
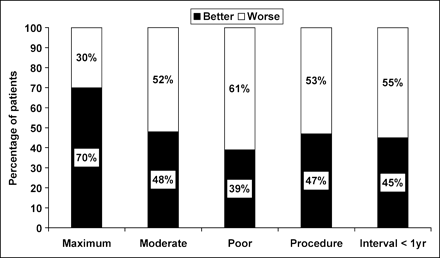
In Figure 4, the MCh, compared with its baseline, improved in 70% of patients on maximal treatment, in 48% of patients on moderate treatment, in 39% on poor treatment and in 47% of the patients undergoing revascularization procedures (P < 0.001).
Percentage of patients in whom the quadrant pair with the maximal change from baseline to follow-up (MCh) improved (better) or became more severe (worse) for each treatment group (P < 0.001).
The FuW, when compared with BW improved in 66% of patients on maximal treatment, in 42% on moderate treatment, in 39% on poor treatment, and in 51% of the patients undergoing revascularization procedures. As noted, FuW was different from BW, reflecting development of a new, more severe lesion, in 84 of 409 or 21% of patients. Of these 84 patients, 8 (9%) were on maximal treatment, 37 (26%) on moderate treatment, and 23 (25%) on poor medical treatment, as shown in Figure 5. New, more severe lesions developed in 22% of the patients who underwent coronary revascularization and in 16% of those who had <1-y interval between PET scans (Fig. 5). These data indicate that intensely treated patients had fewer new flow-limiting stenoses compared with the poorer treated patients (P = 0.01) at follow-up PET.
Percentage of patients with the FuW in a different region than at baseline that was also more severe than at baseline for each treatment group (P = 0.01).
In Figure 6, the BW at follow-up improved in 70% of the patients on maximal treatment, in 48% on moderate treatment, and in 42% on poor treatment (P = 0.02). Of all patients who underwent revascularization procedures, BW improved in 60%, comparable to maximal medical treatment (P = 0.50); there were no significant changes in 55 of 326 or 16.8% of patients.
Percentage of patients in whom the BW became better, worse, or showed no change in each treatment group (P = 0.02).
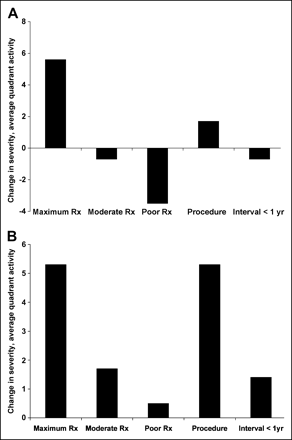
Figure 7 shows the quantitative change as a continuous variable of severity (average quadrant activity) from baseline–to–follow-up PET for the quadrant pair with maximum change from baseline to follow-up, MCh (Fig. 7A), and for the quadrant with the worst baseline defect, BW (Fig. 7B), for each treatment group. The maximally treated patients showed highly significant improvement in perfusion versus the less intensely treated groups (P = 0.001). In the poor treatment group, the worst baseline (BW) defect did not become more severe at follow-up PET but, instead, there was worsening in other quadrants of the heart. These analyses of continuous values for myocardial perfusion changes from baseline–to–follow-up PET provide a different viewpoint and statistical approach that lead to the same conclusions as the analysis of discrete endpoints as the percentage of patients as shown in Figure 4.
Quantitative change in severity (average quadrant activity) from baseline–to–follow-up PET for the quadrant pair with maximum baseline–to–follow-up change, MCh (A), and for the quadrant with the worst defect at baseline, BW (B). Vertical axis values are mean delta or mean change in relative normalized activity expressed as % of maximum as defined in the text, not 2% change (P ≤ 0.001 for A and B).
After a revascularization procedure, the severity of the BW improved as expected, comparable to the improvement noted after maximal medical treatment (Fig. 7B). These findings are consistent with data we previously reported (18) as follows: the integrated combined size–severity of perfusion defects throughout the whole heart at follow-up decreased (improved perfusion) by −21.7% in the maximal treatment group, increased (worsened perfusion) by +3.1% in the moderate treatment group, increased substantially by +23.9% in the poor treatment group, increased minimally by +1.7% in the group with <1y interval between PET scans, and increased by +12.6% in the revascularization group, with P = 0.0001 for group differences.
The changes in severity of the BW from baseline to follow-up (BW) first reported here in this study parallel the changes in combined size–severity of perfusion defects for each of the treatment groups previously reported, except for the revascularization group. In the revascularization group of 45 patients, the quantitative severity of the BW decreased (improved) by 5.2% from baseline values, whereas the combined size–severity integrated throughout the whole heart increased (worsened) by 12.6%. Therefore, the expected improvement in severity of the worst baseline defect after revascularization was not paralleled by improvement throughout the whole heart. The revascularization group of 45 people is not large enough for analysis by intensity of medical treatment as maximal, moderate, or poor. Such analysis would also be biased by the effects of revascularization, irrespective of the intensity of medical treatment, if analyzed as a single or combined group. The differences among the treatment groups, including the revascularization group, for all continuous quantitative baseline–to–follow-up comparisons were significant (P ≤ 0.001).
In Table 4, multivariate logistic regression analysis adjusted for baseline size and severity of the perfusion defects of 15 clinical variables and 6 PET endpoints identified the following independent variables to be predictors of cardiovascular events over 5-y follow up: diabetes, family history of CAD, LDL cholesterol, HDL cholesterol, combined intense lifestyle and pharmacologic treatment, statin treatment, niacin or fibrate treatment, exercise, baseline–to–follow-up change in combined size–severity (odds ratio, 3.3; CI, 2.1–5.5; P = 0.01) and the Combined Quadrant changes (odds ratio, 2.6; CI, 1.7–3.1; P = 0.02), both of which accounted for the integrated overall changes throughout the heart. The change in the BW or any single quadrant pairing did not predict outcomes (Table 4). By Pearson correlation analysis, size or severity alone was not significantly correlated with either combined size–severity or Combined Quadrant changes.
PET Perfusion Changes at Follow-up as Predictors of Long-Term Cardiovascular Events by Stepwise Multivariate Logistic Regression Analysis
DISCUSSION
To our knowledge, this study is the first to demonstrate by noninvasive myocardial perfusion imaging the mechanisms of progression or regression of coronary atherosclerosis related to intensity of risk factor treatment and long-term risk of coronary events. At follow-up PET in our study, most of the patients, 77%, had the greatest sequential perfusion changes in areas of the heart different from the baseline most severe flow-limiting stenosis, 40% had a new flow-limiting stenosis in an area other than the baseline worst perfusion defect, and 21% developed a new perfusion defect worse than the baseline severity in an area other than the baseline worst perfusion defect.
Measures of the net overall perfusion changes throughout the entire coronary vascular tree, including regions other than the worst baseline stenosis, were predictors of coronary events but the change in the worst flow-limiting stenosis at baseline or any one segment of myocardium were not. Finally, patients undergoing intense lifestyle and pharmacologic treatment had more frequent regression of the baseline worst flow-limiting stenoses, fewer new perfusion defects and more frequent perfusion improvement in areas other than the baseline worst perfusion defect. The perfusion images reflect the balance of changes in anatomic stenosis, diffuse disease, and vasomotion due to endothelial dysfunction or their improvement with differing intensities of risk factor treatment not accounted for by arteriography or intravascular ultrasonography.
The change in severity of the BW was not significantly correlated with either the combined size–severity or Combined Quadrant changes by Pearson correlation analysis, consistent with the failure of changes in the BW or of any single quadrant pairing to predict clinical events. Thus, the changes accounting for the net balance of improving or worsening perfusion throughout the heart were better independent predictors of events than the change of the worst baseline flow-limiting stenosis. As expected and previously demonstrated (18), clinical risk factors and treatment intensity were also predictors of outcomes paralleling the integrated PET changes.
The regression or progression of coronary atherosclerosis throughout the entire coronary vascular tree as measured by changes in myocardial perfusion over a wide range of risk factor treatment provides direct evidence on the mechanism for the disproportionately greater reduction in cardiac events than changes in single stenosis severity in prior lipid-lowering trials using arteriographic endpoints (3,4,10–13). Inadequate risk factor treatment is associated with modest worsening in severity of the region with the worst baseline flow-limiting stenosis and a proportionately much greater frequency of new perfusion defects due to new stenosis in regions other than the worst region at baseline in association with more coronary events. This progression in areas other than the worst baseline stenosis also explains why the culprit artery in myocardial infarction often shows only mild stenosis on a prior arteriogram (7).
The group with revascularization procedures provides interesting information for the following reasons: (i) this group shows changes in regions other than the quadrant with the most severe baseline perfusion defect that subsequently underwent revascularization; (ii) the improved perfusion after revascularization in the quadrant with the most severe perfusion defect was no greater than the improvement in patients undergoing intense medical treatment; and (iii) the revascularization group serves as an internal control that tests whether PET perfusion images show improvement as they should after revascularization following the baseline PET.
Although the BW improved with intense risk factor treatment and worsened in its absence, the changes in the BW did not predict outcomes. In contrast, combined perfusion changes throughout the heart did predict outcomes, thereby indicating that the benefits of intense risk factor treatment are largely due to prevention of progression and plaque rupture in regions of the heart other than the baseline worst flow-limiting stenosis.
The current article provides new data on the mechanism for the disproportionately greater effects of lipid management on clinical outcomes than changes in single stenosis severity as assessed by coronary arteriography. Randomization of subjects to treatment groups is not necessary for this study on the mechanisms of progression or regression associated with clinical outcomes where a wide range of treatment intensity is essential to the analysis. Absolute myocardial perfusion in mL/min/g was not used in our study because quantification of relative myocardial uptake is optimal for the hypothesis tested without the assumptions and complexity of serial uptake images, arterial input imaging, and calculated absolute perfusion (25).
CONCLUSION
In patients with CAD undergoing sequential dipyridamole PET perfusion imaging and varying intensity of risk factor treatment with complete long-term follow-up for clinical events, 77% of the patients had the greatest perfusion changes in areas different from the baseline most severe flow-limiting stenosis and 40% developed a new perfusion defect in areas different from the baseline worst perfusion defect. The changes in perfusion defects throughout the entire coronary vascular tree in regions other than the worst baseline stenosis predicted coronary events, whereas the change in the worst flow-limiting stenosis at baseline or in any single, one segment of myocardium did not. These changes in myocardial perfusion throughout the entire coronary vascular tree over a wide range of risk factor treatment intensities provide direct evidence on the mechanism for the disproportionately greater reduction in cardiac events than the changes in single stenosis severity in prior lipid-lowering trials using arteriographic endpoints.
References
- Received for publication July 27, 2005.
- Accepted for publication September 27, 2005.