Abstract
The value of PET or PET/CT with 18F-FDG for the staging of penile cancer has yet to be determined. The objective of this study was to investigate the pattern of 18F-FDG uptake in the primary malignancy and its metastases and to determine the diagnostic value of 18F-FDG PET/CT in the staging and restaging of penile cancer. Methods: Thirteen patients (mean ± SD age, 64 ± 14.0 y) with suspected penile cancer or suspected recurrent disease were examined with a Gemini PET/CT system (200 MBq of 18F-FDG). The reference standard was based on histopathologic findings obtained at biopsy or during surgery. Results: Both the primary tumor and regional lymph node metastases exhibited a pattern of 18F-FDG uptake typical for malignancy. Sensitivity in the detection of primary lesions was 75% (6/8), and specificity was 75% (3/4). On a per-patient basis, sensitivity in the detection of lymph node metastases was 80% (4/5), and specificity was 100% (8/8). On a nodal-group basis, PET/CT showed a sensitivity of 89% (8/9) in the detection of metastases in the superficial inguinal lymph node basins and a sensitivity of 100% (7/7) in the deep inguinal and obturator lymph node basins. The mean ± SD maximum standardized uptake value for the 8 primary lesions was 5.3 ± 3.7, and that for the 16 lymph node metastases was 4.6 ± 2.0. Conclusion: According to our results, the main indication for 18F-FDG PET in the primary staging or follow-up of penile cancer patients may be the prognostically crucial search for lymph node metastases. With the use of a PET/CT unit, the additional information provided by CT may be especially useful for planning surgery. Implementing 18F-FDG PET and PET/CT in future staging algorithms may lead to a more precise and stage-appropriate therapeutic strategy. Furthermore, invasive procedures with a high morbidity rate, such as general bilateral lymphadenectomy, may be avoided.
In Europe and the United States, malignant lesions of the penis, measured as a percentage of all types of cancer in males, amount to only 0.4%–0.6%, and these tumors are responsible for less than 1%–2% of all deaths (1,2). In comparison, they are much more common in developing countries, such as Puerto Rico, Uganda, and even China. In these countries, carcinoma of the penis represents up to 10%–22% of all malignancies in males (1).
The overall prognosis of penile carcinoma depends on the involvement of regional lymph nodes (2,3). To date, noninvasive N and M staging has depended on ultrasound, CT, and MRI. These imaging methods, however, have not proven sufficiently robust in the detection of metastases (1). In many cases, therefore, patients have undergone invasive procedures, such as sentinel lymph node biopsy (SLNB) or general lymphadenectomy, a procedure that is associated with a high morbidity rate. Obviously, patients would benefit from a diagnostic protocol that is noninvasive yet robust and that is able to detect regional lymph node metastases and possible distant metastases with high degrees of sensitivity and specificity.
PET has been shown, in principle, to be a suitable complementary procedure to conventional morphologic imaging methods for the TNM staging of several malignant entities (4). A PET/CT scanner recently was introduced to partially address the poor degree of anatomic resolution of PET by adding high-resolution anatomic imaging provided by CT. This innovative imaging technique, with the radiopharmaceutical 18F-FDG, could prove useful in the noninvasive imaging of penile cancer.
It is known that squamous cell carcinomas often exhibit a high level of 18F-FDG uptake in relation to normal tissue and, hence, may be amenable to diagnosis by PET (5). In over 95% of cases, penile carcinoma is found histologically to be squamous cell carcinoma (1). The objective of the present prospective clinical study was to investigate for the first time whether penile carcinoma exhibits a sufficiently high level of 18F-FDG uptake to be amenable to diagnosis by means of PET. In addition, the study examined the performance of 18F-FDG PET/CT in the TNM staging of this malignancy, including the prognostically crucial determination of patients’ lymph node status.
MATERIALS AND METHODS
Patient Collective
Included in the present study were 13 patients with a mean ± SD age of 64 ± 14.0 y (range, 25–81 y). For 10 patients, examinations were conducted on the basis of a high degree of suspicion of penile cancer after clinical inspection. The 3 remaining patients included in the study presented for follow-up after resection or partial amputation of the penis secondary to penile cancer. After examination by palpation, inguinal lymph node metastases were suspected in 1 of these patients. In the second patient, a previously performed MRI examination aroused suspicion of local disease recurrence at the site of resection. In the third patient, complete resection of a T1-stage tumor was doubtful on the basis of histopathologic examination. One other patient was examined a second time by 18F-FDG PET/CT because of clinical suspicion of local recurrence of lymph node metastases after radical bilateral lymphadenectomy. Both PET/CT scans were included in the data analysis. The human study was approved by the Institutional Review Board of the University of Munich and was performed in accordance with the ethical standards described in the Declaration of Helsinki. Informed consent of the patients was obtained before inclusion in the study.
18F-FDG PET/CT
18F-FDG PET/CT was performed for whole-body staging. Patients fasted for at least 6 h before the examination to maintain serum glucose concentrations below 120 mg/dL. All examinations were performed with a Gemini PET/CT system (Philips), consisting of a dedicated germanium oxyorthosilicate full-ring PET scanner and a dual-slice helical CT scanner. After intravenous injection of 18F-FDG (200 MBq), patients received 20 mg of furosemide to accelerate renal 18F-FDG elimination and 20 mg of butylscopolamine bromide to decrease intestinal 18F-FDG uptake (6). All patients underwent placement of an indwelling urinary catheter to prevent collection of urine, which could compromise visualization of the lower pelvis. The catheter was fixed with adhesive tape, so that the penis was oriented in the upright position. At 60 min after the application of the tracer, low-dose CT (20 mA, 140 kV, 512 × 512 matrix) covering the area from the base of the skull to the proximal thighs was performed. According to our standard clinical protocol, this scan was used for the purpose of attenuation correction, thus avoiding possible artifacts related to intravenous contrast medium. Thereafter, emission measurement was conducted caudocranially in the 3-dimensional mode with a 144 × 144 matrix. Emission scan time per bed position was 3 min; 10 bed positions (field of view, 155 mm) were acquired. After the emission scan, a diagnostic CT scan of the thorax, abdomen, and pelvis was performed after automated intravenous injection of 120 mL (2.5 mL/s) of iodine-containing contrast medium (Ultravist 300; Schering). Scan delay after injection was 50 s to depict the venous phase. Diagnostic dual-slice CT with an axial field of view of 600 mm and a matrix of 512 × 512 was performed in the spiral mode under continuous acquisition at 120 kV and 145 mA (slice thickness of 5.0 mm, increment of 2.5 mm/s, rotation time of 0.5 s, and pitch index of 1). The average total PET/CT examination time was 40 min.
Data Reconstruction, Standardized Uptake Value (SUV) Measurement, and Statistical Analysis
After scatter and decay correction, PET data were reconstructed iteratively with and without attenuation correction and reoriented in axial, sagittal, and coronal slices. A fully 3-dimensional reconstruction algorithm based on the row action maximum-likelihood algorithm was used with PETView software (Philips) (7,8). According to our routine protocol, 2 iterations and a relaxation parameter of 0.0035 were used. In addition, multiplanar reconstructions of the CT data were performed. Manually defined polymorphic regions of interest were drawn on the attenuation-corrected PET image throughout all axial planes in which a suspected lesion could be delineated, tightly including the focus of increased 18F-FDG uptake. For this procedure and for qualitative image analysis, Syntegra software (Philips) was used. The maximum SUV (SUVmax) was documented in an Excel database (Microsoft) for each lesion. The program SPSS version 11.0 (SPSS Inc.) was used for statistical treatment of the data. Means are presented with 1 SD. The Mann–Whitney U test for 2 independent samples was performed to compare the SUVmax of grade 2 and the SUVmax of grade 3 primary tumors. The maximum diameter of all lymph node metastases was assessed by CT, and lesions smaller than 2.5 cm were compared with their corresponding SUVmaxs by linear regression analysis. In addition, a 2-tailed test for significance of the Pearson correlation coefficient was performed. Two-tailed P values of <0.05 were considered significant.
Reference Standard
The reference standard used in this study is based on histopathologic findings obtained at biopsy or during surgery. Surgical procedures included excision of the primary malignancy, partial amputation of the penis, and radical bilateral lymphadenectomy. The 1997 TNM staging system was used to determine the pathologic stage of the primary tumor and its metastases (9). Intraoperative resection specimens were sent for frozen-section examination and afterward for final histopathologic examination, consisting of routine paraffin-section examination, hematoxylin–eosin staining, and immunohistochemical staining. In 3 patients with histopathologically confirmed T1 G2 primary malignancies, lymphadenectomy was not performed because of tumor stage and grade. After surgical resection of the primary tumor, these patients were monitored for a follow-up period of at least 6 mo. During that interval, MRI of the pelvis, ultrasonography of the inguinal region, and clinical examinations were performed, including manual palpation and tumor marker testing for squamous cell carcinoma antigen. The findings of 18F-FDG PET/CT were used as a guide during bilateral lymphadenectomy.
Study Design
All examinations were performed prospectively and interpreted by investigators aware of the patient’s underlying disease but unaware of the results of other diagnostic imaging methods or any other clinical information. Initially, PET images were read independently from CT images by a certified nuclear medicine physician with over 10 y of PET experience. Evaluation of PET images included both attenuation-corrected and noncorrected images. The interpretation of a lesion as benign or malignant was based primarily on a visual analysis. SUVmaxs served solely as guidance. After the PET reviews, fused PET/CT images were evaluated in consensus with a radiologist with over 3 y of CT experience. Lesions classified as malignant by PET maintained a malignant status when the corresponding CT image revealed a morphologic correlate, for example, a lymph node abnormality. Any abnormalities seen on CT without corresponding increased 18F-FDG uptake were interpreted as nonmalignant. We did not aim to compare the diagnostic efficacy of PET/CT with that of stand-alone PET or CT. Therefore, the results presented are based primarily on the information provided by PET, and CT was used mainly for exact anatomic localization. For this purpose, precise image fusion, as provided by the PET/CT system, was of great importance.
RESULTS
Primary Lesions
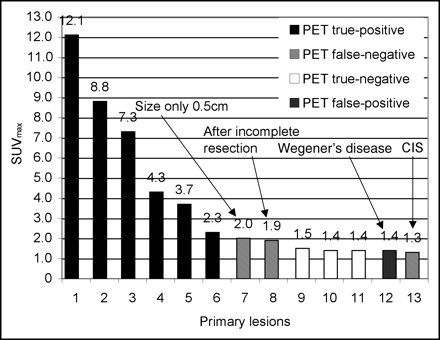
All primary malignancies were squamous cell carcinomas located on the glans penis. In 8 of 13 patients, penile cancer was confirmed by histopathologic examination. Six of these 8 cases showed intense 18F-FDG uptake and were diagnosed as true-positive cases by 18F-FDG PET/CT (Fig. 1). Five of these 8 patients had grade 2 lesions, whereas 3 of 8 had grade 3 lesions. The maximum diameter of primary lesions, as assessed during surgery, ranged from <0.5 cm to 3.5 cm. PET/CT results were interpreted as false negative in 2 of 8 patients. In 1 of these 2 patients, a T1 G2 tumor with a maximum diameter of 0.5 cm was missed. The second patient was referred to our hospital for reexcision after incomplete resection of a T1 G2 tumor at another clinic. Retrospectively, in both cases, a discrete increase in 18F-FDG uptake corresponding to the lesion could be delineated. However, the 18F-FDG uptake pattern was falsely interpreted as nonmalignant during image analysis. In 1 of 13 patients, histologic examination revealed carcinoma in situ (CIS). The tumor did not penetrate the basilar membrane, and PET/CT revealed no pathologic findings.
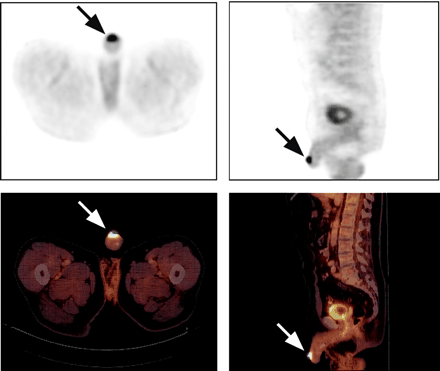
Axial (left) and sagittal (right) PET (top) and PET/CT (bottom) images of 66-y-old man with cancer at glans penis (arrows). 18F-FDG uptake is intense. Histopathologic examination revealed T2 G3 tumor with maximum diameter of approximately 3 cm.
Histopathologic examination revealed no signs of malignancy in 4 of 13 patients. PET/CT results were interpreted as false positive in 1 of these 4 patients, who showed slight 18F-FDG uptake at the glans penis, with an SUVmax of 1.4. Because of its focal character, this lesion was interpreted as malignant. However, after histopathologic examination, the lesion was found to be caused by inflammatory changes attributable to Wegener’s disease. PET/CT results were interpreted as true negative in 3 of these 4 patients. One of these patients had partial necrosis of the glans penis caused by peripheral arterial occlusive disease resulting from diabetes mellitus. On clinical inspection, this lesion was mistaken for penile cancer. PET/CT showed no increased 18F-FDG uptake at the site of necrosis. In the other 2 patients, who had a history of partial amputation of the penis, 18F-FDG PET/CT showed no pathologic 18F-FDG uptake at the resection site, although MRI aroused suspicion of local recurrence in 1 patient. Biopsy, however, revealed no malignancy.
On the basis of our data for this selected patient population, 18F-FDG PET/CT showed a sensitivity of 75% (6/8) and a specificity of 75% (3/4) in the detection of primary lesions. CIS was not included in these calculations because malignancies at this stage are generally not detectable by either PET or conventional morphologic imaging techniques.
Metastases
In 5 of 13 patients, histopathologic examination revealed lymph node metastases, which were localized bilaterally in 2 patients. One of these patients underwent a second PET/CT scan after radical bilateral lymphadenectomy. He developed recurrence of the metastatic spread in the right inguinal and iliac region. Both PET/CT examinations of this patient were included in the data analysis. A total of 16 lymph node metastases were verified by histopathologic examination in these 5 patients. Nine metastatic lesions were located in the superficial inguinal region, and another 5 lymph node metastases were found in the deep inguinal lymph node basins. Two more lesions were located in the obturator region. PET/CT results were interpreted as true positive for all lymph node metastases located in the deep inguinal and obturator lymph node basins, and there was a single false-negative finding in 1 of the 9 superficial inguinal lesions. In total, PET/CT identified 15 of 16 lymph node metastases as true positive.
The majority of the metastatic lesions showed intense 18F-FDG uptake (Fig. 2). All of the foci rated as malignant by PET corresponded to lymph nodes on CT. Three metastatic lymph nodes were ≤1 cm in diameter. The diameter of the single false-negative lesion was 1.8 cm. Despite the pathologic size on CT, the lesion was interpreted as nonmalignant because of the rather discrete 18F-FDG uptake seen on PET, with an SUVmax of 1.1. Intraoperative frozen sections showed no malignancy. However, appropriate histopathologic examination of this lymph node revealed a micrometastasis. This patient had no other metastatic lesions and therefore was inaccurately categorized by PET.
PET (A), CT (B), and PET/CT (C) images of 81-y-old man with history of partial amputation of penis because of T2 G3 penile cancer. 18F-FDG PET/CT revealed multiple lymph node metastases during follow-up. Largest portion of large lymph node metastasis in right inguinal region is necrotic, corresponding to photopenic defect in PET. Only small rim of viable tumor tissue at margin of lesion shows increased 18F-FDG uptake. Cranial to this lesion, smaller lymph node metastases can be seen (arrows).
In 8 of 13 patients, neither clinical findings nor diagnostic imaging procedures aroused suspicion of lymph node metastases. In 3 of these 8 patients, lymph node metastases could be ruled out with respect to the primary lesion (partial necrosis, Wegener’s disease, and CIS). In another 2 patients, a radical bilateral lymphadenectomy was performed and revealed no malignancy. The remaining 3 patients were monitored for a follow-up period of at least 6 mo. PET/CT results were interpreted as true negative in all 8 patients, and there were no false-positive findings.
In summary, 18F-FDG PET/CT results were interpreted as true positive in 15 of 16 lymph node metastases, and there was only 1 false-negative finding corresponding to a micrometastasis. As this was the single metastatic lesion in this patient, sensitivity in the detection of lymph node metastases was 80% (4/5) on a per-patient basis. On a nodal-group basis, PET/CT showed a sensitivity of 89% (8/9) in the detection of superficial inguinal lesions, whereas all lymph node metastases located in the deep inguinal and obturator lymph node basins were identified as true positive, resulting in a sensitivity of 100% (7/7). The sensitivity of PET/CT on a per-lesion basis was 94% (15/16). The results for all 8 patients without metastatic spread were interpreted as true negative; the specificity of PET/CT in the detection of lymph node metastases therefore was 100% (8/8) on a per-patient basis.
SUVs
18F-FDG uptake in histopathologically proven primary malignancies and lymph node metastases was markedly high. The mean ± SD SUVmax of the 8 histopathologically proven malignant lesions (CIS was not included) was 5.3 ± 3.7, with a range of 1.9 to 12.1. When grade 2 tumors are compared with grade 3 tumors, a tendency is visible: grade 3 lesions show higher 18F-FDG uptake (mean ± SD SUVmax, 7.9 ± 3.9) than grade 2 tumors (mean ± SD SUVmax, 3.7 ± 2.9). However, it may be speculated that because of the small sample size, this difference was not significant (P = 0.143). The SUVmaxs of benign lesions (necrosis and Wegener’s disease) and of regions of interest drawn around the distal part of the penis after partial amputation were ≤1.5 (Fig. 3). With regard to lymph node metastases, the mean ± SD SUVmax of the 16 lesions was 4.6 ± 2.0, with a range of 1.1 to 7.6. In 3 lymph node metastases, the SUVmax was lower than 3.0. In 1 of these cases, a lesion with an SUVmax of 1.1 was found to be a micrometastasis. In the other 2 cases, the lymph node metastases had maximum diameters, as assessed by CT, of less than 1 cm, with SUVmaxs of 1.3 and 2.8. This effect is known to occur with SUV quantification of smaller lesions because of partial-volume effects caused by the limited spatial resolution of PET.
SUVmaxs of primary lesions and 18F-FDG PET/CT findings.
DISCUSSION
The diagnosis of penile carcinoma is most often established by biopsy of the visually inspected area of suspicion. With respect to lymphogenous metastasis, Solsona et al. have reported a correlation between increasing stage and grade and the occurrence of lymph node metastases (10). We have been able to confirm this observation in our own patient collective. Of 5 patients with confirmed lymphogenous metastasis, 4 patients were staged at T2 G3 and only 1 was staged at T1 G2. Distant metastases occur in less than 10% of cases and are generally associated with regional lymph node involvement (2).
Despite adequate treatment, which is based primarily on surgical procedures, further progression of the disease cannot be prevented in up to 40% of patients (11). The overall prognosis of penile carcinoma depends on both T staging and grading of the primary tumor, together with the presence or absence of lymphogenous metastasis (3,12). The noninvasive evaluation of possible lymph node involvement begins with palpation and is supplemented with cross-sectional imaging techniques, such as ultrasound, CT, and MRI (13). Ultrasound is considered unreliable with regard to N staging (14). The other cross-sectional radiologic imaging techniques also have proven disappointing in evaluating patients for possible regional lymph node involvement (1). For example, Lont et al. described frequent false-positive findings with CT and MRI (15). One reason for this phenomenon is that lymph nodes undergo reactive changes secondary to malignant disease in the penis (3). Furthermore, even under physiologic conditions, the diameter of lymph nodes in the inguinal region can exceed 2 cm in the absence of malignancy. According to Algaba et al., 20%–96% of patients have palpable lymph nodes at the time of first diagnosis, although actual lymphogenous metastasis is present in only 17%–45% of these patients (14). An invasive option for assessing lymphogenous metastasis is SLNB after peritumoral injection of nanocolloid particles that are marked with 99mTc. For some tumor entities, such as mammary cancer, this procedure is known to have high accuracy (16). Tanis et al., however, reported a comparatively low overall sensitivity of 78% in penile cancer (17). Although noninvasive imaging techniques generally should not serve as a replacement for SLNB, this invasive method is not yet considered a standard procedure in the examination of penile cancer patients, as is the case with several other malignancies. The unsatisfactory accuracy of currently available diagnostic methods supports the demand for general, preventive lymphadenectomy (3). Some authors consider this approach to be indicated only for G3 tumors in stages T2–T4 (18). General bilateral lymphadenectomy is associated with a high morbidity rate of 30%–50% (14).
18F-FDG PET has been shown to be particularly useful in assessing lymph node status (4). A disadvantage of the method is its low spatial resolution, which often precludes exact anatomic localization of pathologic changes. These problems, however, can be partially compensated by recently introduced PET/CT scanners. Preliminary studies have shown that PET/CT has, in some cases, resulted in diagnostic accuracy higher than that of the individual techniques (19–22). The objective of the present study was to investigate for the first time the basic suitability of 18F-FDG PET/CT in the diagnosis of penile carcinoma. At present, there are no systematic studies of larger collectives by PET or PET/CT for TNM staging or diagnosis of recurrent disease in patients with penile carcinoma. To our knowledge, the international literature includes only 3 cases that suggest increased 18F-FDG uptake in penile carcinoma. In a case report that focused primarily on a novel polychemotherapy regimen, Joerger et al. reported a metastatic lymph node that was discovered incidentally at follow-up and that showed increased 18F-FDG uptake (23). Ravizzini et al. also described a lesion with positive 18F-FDG uptake in a patient with penile carcinoma (24). Finally, Langen et al. reported a coincidentally discovered metastatic inguinal lymph node that showed increased 18F-FDG uptake (25). In none of these studies was the efficacy of 18F-FDG PET in the diagnosis of penile carcinoma the primary focus.
Our findings in 13 patients show that both the primary tumor and regional lymph node metastases exhibit a pattern of 18F-FDG uptake typical for malignancy. In our selected patient population, 18F-FDG PET/CT showed a sensitivity of 75% (6/8) and a specificity of 75% (3/4) for the group of primary malignancies. With regard to the pattern of uptake of the primary tumor, there was a trend suggesting that the grade of the tumor exerts a certain influence over the intensity of 18F-FDG uptake. Because the primary tumor is very accessible to biopsy and because distant metastases are fairly rare and occur only in very advanced disease stages, the main indication, in our opinion, for 18F-FDG PET and PET/CT is in the area of N staging. For this purpose, we found a high sensitivity of 94% (15/16) on a per-lesion basis and no false-positive findings. PET/CT missed only 1 of 16 lesions, which was found to be a lymph node micrometastasis. On a nodal-group basis, the sensitivity of PET/CT was 89% (8/9) in the detection of metastases in the superficial inguinal lymph node basins. Furthermore, all lesions located in the deep inguinal and obturator lymph node basins were detected as true-positive lesions (7/7). Metastatic spread to the deep inguinal or obturator lymph node basins results in N3-stage disease (9).
Data provided by PET made it possible to assess the malignancy of lymph nodes independent from a system of classification that is oriented primarily on size, as is the case with CT and MRI. The use of PET/CT scanners may prove especially useful in preoperative therapy planning. The information provided by CT allows exact evaluation of the anatomic position of the lesion and neighboring structures. A further future area of application for 18F-FDG PET and PET/CT may be in follow-up.
As has been shown in our collective for 4 metastatic lymph nodes measuring 0.7–1.1 cm in diameter, even small lesions can be detected because of the usually intense pattern of tracer uptake in this type of malignant disease. One of these lesions exhibited a rather low SUVmax of 1.3. At first, this metastatic lesion was incorrectly classified as nonmalignant by reading of the PET image because of discrete tracer uptake. However, on the basis of exact image fusion with CT, which revealed a lymph node with a diameter of 0.8 cm at this site, the status was changed to malignant during consensus reading (Fig. 4). The relatively low SUVmax of this lesion can be explained by a partial-volume effect. We accordingly found a significant positive correlation between SUVmax and the maximum diameter of metastatic lymph nodes up to 2.5 cm in diameter (Pearson correlation coefficient, 0.75; P = 0.019). As is well known, this effect can be minimized by determining scanner-specific calibration factors (26–28). In routine interpretation, however, it is usual to refer to the uncorrected and easily determined SUVmax. Especially for small lesions, this value should be used only for guidance and should not be considered an absolute value.
PET (A), CT (B), and PET/CT (C) images of 61-y-old man with small inguinal lymph node metastasis originating from T2 G3 penile carcinoma. Maximum diameter, as determined by CT, was 0.8 cm. Despite small size, lesion showed focal 18F-FDG uptake (arrows); consequently, PET/CT results were interpreted as true positive.
Because of the low prevalence of this tumor entity, this study was based on a selected patient population, including both initial staging and restaging of patients. This heterogeneous patient group must be considered a limitation of this study. Another limitation may be the use of 18F-FDG PET/CT findings as a guide during bilateral lymphadenectomy. Although this approach ensured resection of all suspected lymph nodes, it also could have biased the underlying reference standard. One intrinsic limitation of 18F-FDG PET and PET/CT is the detection of micrometastases. Although the scanner-specific resolution of modern PET scanners is less than 1 cm for lesions with intense tracer uptake, the detection of smaller micrometastases is usually unsuccessful. In our collective, 1 micrometastasis represented the only false-negative finding in the N staging. Because the methodical approaches of SLNB and PET/CT are different, the application of SLNB parallel to PET/CT may reduce the risk of unrecognized micrometastasis. A combination of both methods may ensure a sufficiently high degree of diagnostic accuracy to dispense with general lymphadenectomy which, although practiced by some centers, is associated with a high morbidity. This scenario is especially possible in cases with low-grade disease and a low T stage.
CONCLUSION
In this selected patient group, the majority of primary malignancies as well as their metastases showed markedly increased 18F-FDG uptake. 18F-FDG PET/CT showed high sensitivity and specificity in the staging and restaging of penile cancer. Morphologic information provided by CT and the possibility of exact image fusion may prove especially useful for planning surgery. We therefore conclude that 18F-FDG PET/CT is of value in the staging of penile cancer. Implementation of PET and PET/CT in future staging algorithms may lead to an increase in diagnostic efficacy and consequently to more precise and stage-appropriate therapeutic regimens. Furthermore, the added diagnostic security provided by PET may help to decrease the need for invasive procedures with a high morbidity rate, such as general bilateral lymphadenectomy, which then may be avoided in cases with a low tumor stage or grade.
Footnotes
Received Feb. 3, 2005; revision accepted May 13, 2005.
For correspondence or reprints contact: Bernhard Scher, MD, Department of Nuclear Medicine, University of Munich, Marchioninistrasse. 15, 81377 Munich, Germany.
E-mail: Bernhard.Scher{at}med.uni-muenchen.de