Abstract
Volumetric blood flow (Q) determination requires simultaneous assessment of mean blood flow velocity and vessel cross-sectional area. At present, no method provides both values. Intracoronary Doppler-based assessment of coronary flow velocity reserve (CFVR) relies on average peak velocity (APV). Because this does not account for changes in velocity profile or vessel area usually occurring with flow-dependent vasodilation, results can be misleading. The aim of this clinical study was to validate against the current gold standard (measurement of myocardial perfusion reserve [MPR] by PET) a new, Doppler-based method for calculating coronary Q and coronary flow reserve (CFR). Methods: Doppler-based intracoronary Q was measured with a proprietary guidewire device in a nonstenotic coronary artery at baseline and during adenosine-induced hyperemic flow (140 μg/kg/min intravenously during 7 min). Three gate positions were assessed, of which 2 were lying within the vessel and 1 was intersecting the vessel. The zeroth (M0) and the first (M1) Doppler moments of the intersecting gate were used to calculate mean blood flow velocity (M1/M0) and vessel area (M0), and M0 of the 2 proximal gates was used to correct for scattering and attenuation. CFR was calculated as hyperemic/resting flow with Q and compared with APV-derived CFVR and with the corresponding segmental MPR obtained with 15O-labeled water and PET. Results: Q (CFR, 2.60 ± 1.07) correlated well with PET (MPR, 2.58 ± 1.11) (r = 0.832, P < 0.005; Bland–Altman limits, −1.42 to 1.09), whereas CFVR did not (r = 0.09, P = not statistically significant; Bland–Altman limits, −3.36 to 2.24). However, in vessels without dilation, there was no difference between CFR, CFVR, and MPR. Conclusion: This procedure for intracoronary Q measurement using the proprietary Doppler guidewire system, which accounts for both changes in flow profile and changes in vessel area, allows invasive, accurate assessment of CFR even in the presence of flow-dependent vasodilation.
Coronary flow reserve (CFR) is defined as the ratio of coronary flow under maximal drug-induced coronary vasodilation to coronary flow under resting conditions. Many studies have documented a decreased reserve in patients at elevated cardiovascular risk, such as smokers (1,2) and individuals with dyslipidemia (3,4), hypertension (5), or diabetes (6).
Because measurement of CFR has gained wide acceptance as a diagnostic and prognostic approach in the clinical decision-making process, accuracy in the measurement is crucial. Accuracy is best achieved using the current gold standard, PET, which provides measurements of myocardial blood flow (MBF, in mL/min/g) and myocardial perfusion reserve (MPR) through division of hyperemic MBF by resting MBF (7). An invasive alternative is assessment of intracoronary volumetric blood flow (Q, in mL/min), which requires simultaneous measurement of vessel cross-sectional area and mean velocity (Vmean). To date, no commercially available system allows measurement of both velocity and area with a single catheter. In daily clinical routine, most interventional cardiologists do not assess cross-sectional area by quantitative coronary angiography because it is time consuming and cumbersome. Coronary blood flow measurements, therefore, rely on blood flow velocity alone, assuming that vessel diameter remains constant during different flow conditions. Similarly, the thermodilution technique for assessing CFR assumes a constant coronary artery diameter (8). However, constancy of diameter does not hold true for coronary arteries, in which flow-induced endothelium-mediated vasodilation may occur. In addition, the commonly used system with a 0.014-inch Doppler wide-beam guidewire (FloWire; Cardiometrics) provides average peak velocity (APV) but not Vmean. For the calculation of Vmean from APV, a constant coefficient of 0.5 is commonly used (Vmean = 0.5 × APV). Unfortunately, this coefficient does not hold true for pulsatile flow (9). Thus, assessment of coronary flow velocity reserve (CFVR) from APV alone suffers from fundamental limitations, may provide misleading results, and therefore must be judged with caution. Because epicardial coronary vasodilation contributes to and participates in increases in coronary blood flow, an accurate invasive means of assessing true increases in myocardial flow requires Q measurement. PET, because it measures nutritive tissue perfusion, is not subjected to the above-mentioned limitations and can serve as a noninvasive gold standard.
We have recently validated in vitro (10), and in vivo in experimental animals (11), a new method for direct measurement of volumetric flow by simultaneous assessment of cross-sectional area and Vmean solely from received Doppler power via FloWire. The aim of the present study was to validate in humans, against PET MPR, our novel technique.
MATERIALS AND METHODS
Study Population
We studied 10 patients (2 women and 8 men; mean age ± SD, 55 ± 12 y) who underwent coronary angiography for suspected coronary artery disease. Four of the patients had no coronary artery disease, and 6 had single-vessel disease for which primary stent implantation had been performed in the right coronary artery (5 patients) or in the left circumflex coronary artery (1 patient). Patients with prior myocardial infarction were excluded from the study. For the intracoronary measurements, a normal coronary artery was chosen (Table 1). We did not choose an artery after stent implantation, because coronary vasomotion, flow, and CFR may be transiently reduced early after stenting and vary over time (12). These effects could have introduced large inaccuracies when the Doppler measurement was compared with PET.
Characteristics of Enrolled Study Population
Study Protocol
All patients were studied first with Doppler and then, within 2–10 d, with PET. Vasoactive medications including calcium-channel blockers, angiotensin-converting enzyme inhibitors, long-acting nitrates, and β-blockers were withheld for at least 24 h before the Doppler and PET studies.
Doppler Measurements
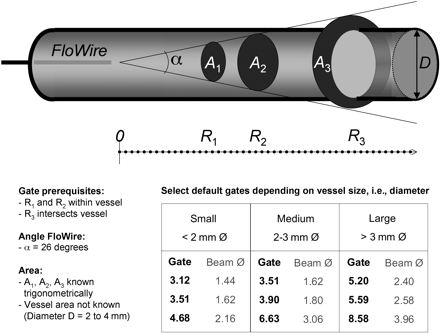
At the end of diagnostic catheterization, intracoronary measurements were performed in the left anterior descending coronary artery (8 patients) or in the left circumflex coronary artery (2 patients) using a FloWire guidewire. At the tip of this steerable guidewire, a 12-Mhz piezoelectric crystal is mounted. The forward-directed ultrasound beam diverges ±13° from its axis as measured (by the manufacturer) at the −6-dB points of the ultrasonic beam pattern (2-way beam width). The guidewire is coupled to a commercially available Doppler system (FloMap; Cardiometrics) into which our specific software for the calculation of the Doppler moments was implemented by the manufacturer following our suggestions (10,11). The sample volume depth can be moved along the beam axis at discrete steps of 0.39 mm. The inset in Figure 1 shows the beam diameters as a function of the gate position (Fig. 1). Measurements were performed at various gate positions. On the one hand, some gates were sampled close enough to the probe for the sample volume to lie completely within the vessel lumen (R1, R2). On the other hand, some distant gate positions (R3) were acquired, at which the beam intersects the vessel and thus completely insonates its cross-sectional area (Fig. 1). The unknown diameter of the coronary artery was assumed to be between 2 and 4 mm, accounting for a resting state and a hyperemic state. In practice, the vessel diameter at rest was estimated from angiography, and the vessel was classified as small (<2 mm), medium (2–3 mm), or large (>3 mm). During adenosine-induced vasodilation, up to a 50% increase in vessel diameter was assumed. For measurements during hyperemia, the distant gate R3 was chosen large enough to ensure that the Doppler beam intersected even the dilated vessel. Doppler signals were sequentially acquired with a single-gate pulsed Doppler beam, which requires 1 heart beat (R-R interval) for each sample measurement. Special care was taken for optimal positioning of the Doppler FloWire to obtain high-quality Doppler spectra. These are characterized by strong signals in the high-velocity range and by a sharply defined envelope. Repetitive scans of consecutive gate positions were obtained at rest and between the fourth minute and the last minute of hyperemia. Hyperemia was induced by intravenous infusion of adenosine over 7 min at a rate of 140 μg/kg of body weight per minute according to standard practice for myocardial perfusion scans (13).
Measurements at gate depths 1 and 2 are used to correct received power of gate 3 for attenuation and scattering. Corrected power received from gate 3 corresponds to cross-sectional area of vessel, and at this position Vmean is calculated as M1/M0. (Adapted with permission of (11).)
PET Scan
MBF was assessed on an Advance positron emission tomograph (GE Healthcare) using 500–700 MBq of 15O-water at rest and repeated during adenosine-induced hyperemia after 10 min to allow for decay of the 15O radioactivity in the body. Three minutes after the start of the adenosine infusion, the hyperemic MBF measurement was started. A 20-min transmission scan was then acquired for attenuation correction of all emission scans (4,14,15).
This study protocol was approved by the Institutional Review Board of the University Hospital Zurich. All subjects gave informed and written consent before the study.
Determination of Volumetric Flow by FloWire
The method has been extensively described and validated both in vitro and, in animal studies, in vivo (10,11). In brief, the received Doppler power equals the zeroth Doppler moment (M0) and is proportional to the insonated area at the respective gate depth (R). In addition, the Doppler power received by the transducer depends on the attenuation function of the medium, the scattering function, and the sample volume size (16). For volumetric flow calculation, 3 Doppler power measurements are necessary: M0 measurements at gate depths R1 and R2 are used to correct the received power of gate R3 for attenuation and scattering. Thus, the corrected power (M0) received from gate R3 corresponds to the cross-sectional area of the vessel and, at this position, Vmean is calculated as M1/M0. Finally, with area × Vmean, volumetric flow (mL/min) can be calculated using Equation 1:
 Eq. 1
Eq. 1
All Doppler moments M0 and M1 obtained at the different gate depths were stored on a laptop computer for further analysis. During the offline processing, only data from those gate–position combinations complying strictly with the mathematic condition in Equation 2 were used to calculate volumetric flow:
 Eq. 2
If Equation 2 is fulfilled, the term area in Equation 1 equals vessel area.
Eq. 2
If Equation 2 is fulfilled, the term area in Equation 1 equals vessel area.
Patients with vasodilation of less than 35% of the luminal area were considered nonresponders with regard to adenosine-induced vasodilation of epicardial coronary arteries (17–19).
PET Image Processing
The sinograms were corrected for attenuation and reconstructed on a Sun Microsystems workstation using standard reconstruction algorithms. On factor images, generated by iterative reconstruction (2,4,14,20,21), regions of interest were drawn within the left ventricle and ventricular myocardium on consecutive image planes. These were projected onto the dynamic H215O images to generate blood and tissue time–activity curves, which were fitted to a single-tissue-compartment tracer kinetic model to give values of MBF (mL/min/g) using the pixelwise model software package (PMOD Technologies GmbH) as previously validated (15,22–24). The left ventricle was subdivided into 16 segments according to the coronary territories following the recommendations of the American Heart Association (25). For the present analysis, the segments were grouped to obtain a value for those segments supplied by the artery, which was assessed invasively (22). The segments were assigned to the respective coronary territory by the interventional cardiologist, who was unaware of the PET result before PET analysis.
MPR, CFR, and CFVR
MPR (relative units) by PET was calculated as the ratio of hyperemic over resting MBF (mL/min/g). CFR (relative units) was calculated as the ratio of hyperemic over resting power-based volumetric (Q) intracoronary Doppler flow (mL/min). CFVR (relative units) was calculated as the ratio of hyperemic over resting coronary blood flow velocity (cm/s).
Statistical Analysis
Mean values are given with their SD. MPR, CFR, and CFVR values were compared using ANOVA statistics for repeated measures. If the value for P was less than 0.05, Scheffé’s procedure was applied. In addition, regression analysis was performed and limits of agreement between the different methods were calculated according to Bland and Altman (26).
RESULTS
Mean values for Doppler-assessed flow velocity and PET-assessed MBF are given in Table 2. MPR was 2.58 ± 1.11 by PET and CFR was 2.60 ± 1.07 by Doppler power measurements (Q). By contrast, CFVR was 1.85 ± 0.84 by Vmean (P < 0.05 vs. PET and Q) and 2.18 ± 0.96 by APV.
Flow Results by PET and by Doppler
A significant correlation was found between PET (MPR) and Doppler power Q (CFR) measurements (r = 0.832; P < 0.005) but not between PET (MPR) and APV (CFVR) or Q (CFR) and APV (CFVR). These results are given in Figure 2, which also provides Bland–Altman plots and limits of agreement.
(Top) Correlation was significant between CFR as assessed by volumetric intracoronary flow measurement (Q) and MPR as assessed by PET, but no correlation existed between CFVR as assessed by APV and PET or between Q and APV. (Bottom) Bland–Altman (BA) limits show good agreement between Q and PET but not between APV and PET or Q and APV. NS = not statistically significant.
Six patients (Table 1) did not show significant adenosine-induced vasodilation (14% ± 13%), and 4 patients did (100% ± 49%, P < 0.01). In the absence of vasodilation, CFR was quite comparable among the different techniques, that is, 2.46 ± 1.27 by PET, 2.37 ± 1.22 by Q, 2.08 ± 0.98 by Vmean, and 2.37 ± 1.21 by APV, with none being statistically significant. By contrast, in vessels with significant vasodilation, CFR was comparable when PET (3.22 ± 0.79) and Q (2.95 ± 0.82) were used but significantly lower when velocity alone was used, that is, Vmean (1.50 ± 0.49, P < 0.05 vs. PET and Q) and APV (1.89 ± 0.36, P < 0.05 vs. PET) (Fig. 3).
Comparison between MPR, CFR, and CFVR. (Left) In subgroup of vessels with adenosine (Ado)-induced vasodilation, CFVR as assessed with Vmean or APV was significantly lower than MPR with PET or CFR by Doppler power Q. (Right) In vessels without Ado-induced vasodilation, no significant difference was found between methods.
DISCUSSION
Our findings suggest that direct volumetric coronary blood flow measurement from received Doppler power using a Doppler FloWire system is feasible in vivo and allows CFR assessment that is accurate and correlates well with the gold standard, that is, MPR measurements by PET. By contrast, there was no correlation between CFVR and MPR or between CFVR and CFR, because coronary arteries may dilate during hyperemic stimulation and cause an increase in coronary flow by the increase in cross-sectional area even though flow velocity can remain unchanged. PET has repeatedly been shown to provide accurate and reproducible MBF measurements, unaffected by flow-related vasodilation, at rest and during hyperemia (14,15,27). Therefore, PET is an established tool to assess coronary endothelial function. To achieve similar accuracy, intracoronary Doppler measurements require volumetric flow assessment. Measurements based on velocity alone (CFVR) may provide misleading results because changes in cross-sectional area are neglected (28).
Because angiographic findings are not able to predict the physiologic relevance of a coronary stenosis (29–31), it has been recognized that CFR assessment provides useful information (32,33) for making clinical decisions on revascularization therapy (34). For CFR or MPRF assessment, hyperemic flow is induced by vasodilator agents such as adenosine or dipyridamole. Vasodilation also provides the basis for the principle of nuclear myocardial perfusion imaging, as lack of perfusion increase in a stenotic segment induces a heterogenic pattern due to relative underperfusion, revealing a coronary artery lesion, which requires revascularization. These changes in coronary vessel diameter are neglected by the invasive conventional velocity assessment. No accurate CFR can be calculated by velocity information alone, without knowledge of the exact vessel size and its changes. Therefore, validation studies for volumetric coronary blood flow measurements have generally been performed using rigid metal stents (35) or tubes (36,37) to avoid the well-known flow-induced endothelium-mediated coronary dilation (38–40), which would affect the relationship between coronary flow velocity and volumetric flow, introducing an error of up to 40% (28,41). This limitation could, at least in part, be addressed by using intracoronary nitroglycerin before the baseline measurements to maximize vasodilation throughout the study (39). Such a step was not feasible during our PET protocol.
In a subgroup of patients without significant adenosine-induced vasodilation, there was no significant difference between MPR, CFR, and CFVR. This finding documents the important confounding effect of vasodilation on CFVR assessment and underlines the strength of PET in allowing assessment of coronary endothelial function regardless of coronary vasomotion, with pharmacologic stimuli or cold pressor testing.
A potential limitation of our technique is that only high-quality Doppler spectra tracings, which require optimal positioning of the FloWire and correct selection of the gate, provide optimal results. This limitation, however, applies even more to the conventional FloWire measurements.
CONCLUSION
We present for, what is to our knowledge, the first time the in vivo validation in humans of a procedure for intracoronary Q measurement using a Doppler FloWire system that is able to account for changes in flow profile and vessel area. This ability is of particular clinical importance because changes in coronary diameter occur with flow-dependent vasodilation. This method provides accurate data on CFR as documented by validation against PET MPR, which represents the noninvasive gold standard.
Acknowledgments
This study was funded in part by grants from the Swiss National Science Foundation (SNSF-professorship grant PP00A-68835 and grant 3100-068368).
Footnotes
Received Mar. 31, 2005; revision accepted Apr. 6, 2005.
For correspondence or reprints contact: Rolf Jenni, MD, MSEE, Echocardiography, Cardiovascular Center, University Hospital, Ramistrasse 100, CH-8091 Zurich, Switzerland.
E-mail: karjer{at}usz.unizh.ch