Abstract
Adenosine is an endogenous modulator of synaptic functions in the central nervous system. To investigate the physiologic and pathologic roles of the adenosine receptors in the human brain, PET is a powerful in vivo technique. In this study, we quantitatively evaluated the distribution of a major subtype A1 adenosine receptor in the human brain by PET with a newly developed radioligand, 8-dicyclopropylmethyl-1-11C-methyl-3-propylxanthine (11C-MPDX). Methods: In 5 healthy volunteers, after PET measurement of the regional cerebral blood flow (rCBF) with 15O-H2O, a 60-min PET scan with 11C-MPDX was performed. The distribution volume (DV) of 11C-MPDX was quantitatively evaluated by Logan’s graphical analysis. Results: 11C-MPDX was taken up at a high level, reaching a peak at 2–2.5 min, followed by a rapid decrease. The unchanged form of 11C-MPDX in plasma was 75% at 60 min after injection. The DV of 11C-MPDX was large in the striatum and thalamus, moderate in the cerebral cortices and pons, and small in the cerebellum. The distribution pattern of 11C-MPDX in the brain was coincident with that of adenosine A1 receptors in vitro, reported previously, but discretely different from that of rCBF. Conclusion: 11C-MPDX PET has the potential for mapping adenosine A1 receptors in the human brain.
Adenosine is present in large amounts in the mammalian brain and plays a role as an endogenous modulator of synaptic functions in the central nervous system. The effects are mediated by at least 4 adenosine receptor subtypes: A1, A2A, A2B, and A3. The 2 major subtypes of receptors—A1 and A2 receptors—have been investigated well in molecular biology, pharmacology, and physiology (1–3). The adenosine A1 receptors exhibit a high affinity for adenosine and inhibit adenylyl cyclase. It is now known that A1 receptors are G protein linked and can act through effectors other than adenylyl cyclase, including potassium channels, calcium channels, phospholipase A2 and C, and guanylyl cyclase (1).
Previous work has established a role for adenosine in a diverse array of neural phenomena, which include regulation of sleep and the level of arousal, neuroprotection, regulation of seizure susceptibility, locomotor effects, analgesia, mediation of the effects of ethanol, and chronic drug use (4). They are mediated by both adenosine A1 and A2A receptors. Therefore, interaction with adenosine metabolism is a promising target for therapeutic intervention in ischemic brain disorders, neurologic and psychiatric diseases such as epilepsy, sleep, movement (parkinsonism or Huntington’s disease), or psychiatric disorders (Alzheimer’s disease, depression, schizophrenia, or addiction) (3,5,6).
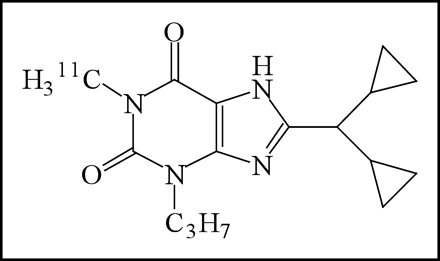
For the purpose of mapping adenosine A1 and A2A receptors in the brain by PET, we synthesized and characterized several radioligands (7,8). Recently we performed imaging of adenosine A1 receptors in the human brain by PET with 8-dicyclopropylmethyl-1-11C-methyl-3-propylxanthine (11C-MPDX) (Fig. 1) (9) following the developmental studies with animals (7,10–15). Bauer et al. also reported the mapping of adenosine A1 receptors in the human brain by PET with a xanthine-type analog, 8-cyclopentyl-3-(3-18F-fluoropropyl)-1-propylxanthine (18F-CPFPX) (16). In this study, we quantitatively evaluated the distribution volume (DV) of 11C-MPDX in the brain of healthy volunteers by a graphical analysis method.
Chemical structure of 11C-MPDX.
MATERIALS AND METHODS
The study protocol was approved by the Institutional Ethical Committee. Five male volunteers (age, 21.6 ± 1.5 y old; age range, 20–24 y old; body weight, 62.4 ± 2.3 kg; body weight range, 59–64 kg) participated in this study, and written informed consent was obtained from the subjects. All subjects were healthy according to the history, physical, neurologic, and psychiatric examinations, and MRI study of the brain before the PET study.
Radiosynthesis of 11C-MPDX was performed as previously described (10,14). The injection radioactivity dose was 611 ± 93 MBq (range, 484–700 MBq), and the mass was 15.5 ± 6.9 nmol (range, 9.9–27.5 nmol). The specific radioactivity was 43.4 ± 13.4 TBq/mmol (range, 25.5–55.6 TBq/mmol).
PET Measurement
PET measurement was performed with a SET-2400W (Shimadzu Co.), which acquires 63 slices having 128 ×128 pixels each at a transverse resolution of 4.5-mm full width of half maximum (FWHM) and at an axial resolution of 5.8-mm FWHM. Scanning took place as the subjects laid supine. A venous catheter was inserted into a forearm vein of the subjects for tracer injection, and an arterial catheter was inserted into a distal radial artery under local anesthesia for sampling arterial blood. After positioning the subject’s head in the canthomeatal orientation, a transmission scan was performed with a rotating 68Ga/68Ge line source to correct for the photon attenuation using the attenuation map. Then, 15O-H2O (120–150 MBq) was injected intravenously into the subject for a period of 10 s, and a PET scan was performed for 120 s in a 3-dimensional static mode. Ten minutes later, after the first scan, the subject was given 11C-MPDX for a period of 10 s, and the second PET scan was performed for 60 min in a 2-dimensional dynamic mode (10 s × 6 frames, 30 s × 3 frames, 60 s × 5 frames, 150 s × 5 frames, and 300 s × 8 frames). The tomographic images were reconstructed using a filtered backprojection method and Butterworth filter (cutoff frequency, 1.25 cycle/cm; order, 2). The data were collected in a 128 × 128 × 31 matrix. The voxel size was 2 × 2 × 6.25 mm.
Blood Sampling and Metabolite Analysis
Arterial blood was taken at 10, 20, 30, 40, 50, 60 70, 80, 90, 100, 110, 120, 135, and 150 s and at 3, 5, 7, 10, 15, 20, 30, 40, 50, and 60 min. The whole blood and plasma were measured for radioactivity with a γ-counter and weighed. The time-activity curves were expressed as becquerels per milliliter or the standardized uptake value (SUV) (grams body weight × Bq/mL tissue/total injected dose). The unchanged form of 11C-MPDX in the plasma sampled at 3, 10, 20, 30, 40, and 60 min was analyzed by high-performance liquid chromatography (HPLC) as described (10).
Kinetic Analysis
PET images were registrated and resliced to MRI with the Ardekani image registration algorithm (17) by UNIX workstations (Silicon Graphics Inc.) using the Dr. View image analysis software system (Asahi Kasei Joho System). Regions of interest (ROIs) were placed on the frontal, medial frontal, temporal, medial temporal, parietal, and occipital cortices, striatum, thalamus, cerebellum, and pons based on MRI. The ROI in the frontal cortex had 835 voxels and that in the pons had 87 voxels (1 pixel = 2 × 2 × 6.25 mm). The pixel numbers in other regions were in-between. The regional cerebral blood flow (rCBF) was measured by the autoradiographic method (18). The binding of 11C-MPDX was evaluated by a graphical analysis according to the method described by Logan et al. (19). Time-activity curves for each ROI of the brain were calculated as becquerels per milliliter or SUV. Using the time-activity curves of the brain tissues and the metabolite-corrected time-activity curve of plasma, the DV for 11C-MPDX was evaluated. Equation 1 describes the concept of the Logan plot in which integrals of the elapsed-time time-activity curve and the plasma time-activity curve normalized by the elapsed-time time-activity curve have a linear relationship and its gradient derives a total DV:
 Eq. 1 where Cp is activity in plasma, t is elapsed time, and int is integral. A Logan plot was applied to every voxel to make a parametric image on the DV. First, the integrated values were calculated using trapezoidal integration, and Logan plots were formed with an integrated plasma time-activity curve. Then, regression lines were estimated using the plots between 10 and 40 min after 11C-MPDX injection. An estimated gradient means total DV, and an image of the DV can be obtained.
Eq. 1 where Cp is activity in plasma, t is elapsed time, and int is integral. A Logan plot was applied to every voxel to make a parametric image on the DV. First, the integrated values were calculated using trapezoidal integration, and Logan plots were formed with an integrated plasma time-activity curve. Then, regression lines were estimated using the plots between 10 and 40 min after 11C-MPDX injection. An estimated gradient means total DV, and an image of the DV can be obtained.
RESULTS
Figure 2 shows the time-activity curves of whole blood and plasma after injection of 11C-MPDX. The SUVs of blood and plasma decreased rapidly for the first 10 min after injection and then decreased gradually. A discrepancy between 2 levels was observed on and after 10 min. The level of blood was slightly higher than that of plasma. Labeled metabolites of 11C-MPDX in plasma were analyzed by HPLC. Three polar metabolites (retention times: 1.8, 3.2, and 4.3 min) were found in addition to 11C-MPDX (retention time: 6.3 min). The unchanged form of 11C-MPDX in plasma was 98.5% ± 0.64% at 3 min, 88.8% ± 2.85% at 10 min, 82.8% ± 5.38% at 20 min, 82.2% ± 8.28% at 30 min, 77.1% ± 5.38% at 40 min, and 74.9% ± 5.00% at 60 min.
Time-activity curves in blood and plasma after injection of 11C-MPDX. Data represent means of 5 subjects. SUVs of blood and plasma decreased rapidly for first 10 min after injection. SUV of blood was slightly higher than that of plasma.
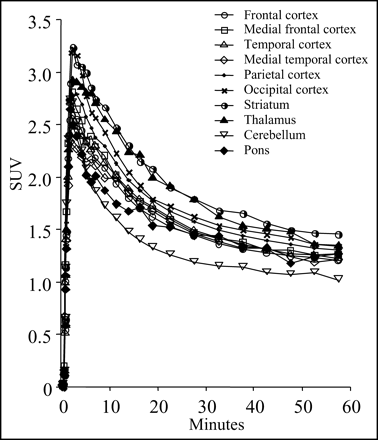
Figure 3 shows the time-activity curves of each brain region after injection of 11C-MPDX. 11C-MPDX was taken at high levels in all regions investigated, and the uptake reached a peak at 2–2.5 min after injection, followed by a rapid decrease for the first 15 min. Approximately 6% of the total injected 11C-MPDX was taken up in the brain at 2–2.5 min. The clearance patterns differed slightly among each region. The SUV was relatively high in the striatum and thalamus and low in the cerebellum.
Time-activity curves in each region in brain after injection of 11C-MPDX. Data represent means of 5 subjects. Uptake of 11C-MPDX in each region was high, followed by rapid decrease for first 15 min. SUV was relatively high in striatum and thalamus and low in cerebellum.
Figure 4 shows the brain MRI and PET parametric images in a typical case. The distribution pattern of 11C-MPDX was discretely different from that of rCBF. The binding of 11C-MPDX was very low in the cerebellum and high in the striatum and thalamus, where the rCBF was relatively lower.
Brain MRI and PET parametric images in typical case. (Top) MRI. (Middle) Distribution image (DV) of 11C-MPDX. (Bottom) rCBF with 15O-H2O. DV of 11C-MPDX was low in cerebellum and high in striatum and thalamus, where rCBF was relatively lower.
Table 1 summarizes the DV of 11C-MPDX and rCBF. A typical Logan plot was shown in Figure 5. The DV was large in the striatum and thalamus, moderate in 6 cortical regions and the pons, and small in the cerebellum. The DV pattern of 11C-MPDX in the brain was different from the rCBF pattern. The rCBF was low in the temporal and medial frontal cortices and pons.
Graphical analysis of 11C-MPDX in frontal cortex, striatum, and cerebellum using Logan plot in 1 case. (•), Data used for linear regression analysis. Slopes of fits represent DVs. C = activity in tissue; Cp(t) = activity in plasma; t = elapsed time.
DV of 11C-MPDX and rCBF in Brain Regions of Young Healthy Subjects
DISCUSSION
Following the preclinical studies on the mapping of adenosine A1 receptors in the brain of cats and monkeys by 11C-MPDX PET (1–15), we performed a clinical study on 11C-MPDX PET and demonstrated the potential of 11C-MPDX as a radioligand for mapping adenosine A1 receptors in the human brain (9). In the present study, we quantitatively evaluated the binding of 11C-MPDX in the brain of healthy subjects.
In the literature, the adenosine A1 receptors are rich in the hippocampus, cerebral cortex, thalamic nuclei, and basal ganglia of the postmortem human brain (16,20,21). In the cerebral cortex, the adenosine A1 receptors were rich in primary visual cortex layer III in the occipital cortex and superficial and intermediate layers in the parietal cortex. On the other hand, the density was relatively low in the frontal, temporal, and cingulate cortices. In the present PET study, the binding of 11C-MPDX evaluated quantitatively as the DV was relatively higher in the striatum and thalamus among the brain regions investigated and lower in the cerebellum. In the cerebral cortex, the DV was relatively larger in the occipital and parietal cortices and smaller in the frontal and temporal cortices. The DV pattern of 11C-MPDX in vivo was consistent with that of the A1 receptor in vitro represented in previous reports (20–24), demonstrating that 11C-MPDX is a suitable radioligand for mapping adenosine A1 receptors.
In the present study, we did not directly certify the specific binding of 11C-MPDX to the adenosine A1 receptors by blocking studies. However, we previously confirmed the A1 receptor-specific binding of 11C-MPDX in animals. Coinjection of unlabeled MPDX or A1-selective KF15372 significantly blocked the brain uptake in mice, rats, and cats (10–13). In a PET study with cats, the reversible binding of 11C-MPDX was clearly demonstrated after treatment with A1-selective 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) (13). In the cat and monkey brains, the regional distribution pattern of 11C-MPDX was similar to that of its analog 11C-KF15372 (13,14), for which the receptor-specific binding was directly confirmed in the monkey brain (11). Furthermore, alteration of 11C-MPDX binding was observed using ex vivo autoradiography in the rat brain treated by monocular enucleation (12) and was observed using PET in the cat brain with cerebral ischemic insult (15). On the basis of these studies, for the quantitative analysis for 11C-MPDX binding in the human brain, we used Logan plot analysis in the present study because it provides relatively stable results compared with the results of kinetic analysis using a 2- or 3-compartment model.
The DV of 11C-MPDX was relatively smaller in the medial temporal cortex including the hippocampus. In postmortem brain studies, the density of adenosine A1 receptor binding sites was heterogeneous, and the density was high in the stratum radiatum/pyramidale of CA1 but low in the stratum moleculare of CA1 and the granule cell layer of the dendate gyrus hilus (20,21). In 11C-MPDX PET, the DV values in these ROIs were assessed as the means of high and low densities of the binding sites, which resulted in the apparent discrepancy between the in vitro findings and 11C-MPDX PET in the medial temporal cortex. The partial-volume effect based on the spatial resolution may be the other reason why the DV in the medial temporal cortex was relatively small.
We performed successively 2 PET scans with 15O-H2O and 11C-MPDX in each subject to verify the influence from rCBF. As shown in Figure 4 and Table 1, the DV pattern was distinctly different from the rCBF pattern. The DV was large in the striatum and thalamus and small in the cerebellum showing a high rCBF. Together with the finding that the DV pattern was consistent with the in vitro receptor distribution pattern in the described postmortem studies (20,21), we concluded that the DV calculated by Logan plot analysis was a stable and sufficient method to estimate the binding of 11C-MPDX to adenosine A1 receptors.
As shown in the time-activity curves of whole blood and plasma (Fig. 2), the radioactivity level in blood was slightly higher than that of plasma. The finding suggests the presence of binding sites or an unknown uptake mechanism for 11C-MPDX in blood cells. With regard to the peripheral metabolism of the tracer, we found that 11C-MPDX was relatively stable in our human subjects compared with the metabolism in experimental animals. The unchanged form of 11C-MPDX in human plasma was 75% of the total radioactivity at 60 min after injection, whereas the corresponding figures were 22%–27% at 30 min in rats (10), 6.5% at 60 min in cats (13), and 41% at 60 min in monkeys (15).
Recently, Bauer et al. also successfully performed imaging of adenosine A1 receptors in the human brain by PET using a similar radioligand, 18F-CPFPX (16). They also evaluated the binding of 18F-CPFPX as the DV. The distribution pattern of 11C-MPDX was consistent with that of 18F-CPFPX. However, for the peripheral metabolism, 11C-MPDX was much more stable than 18F-CPFPX. The unchanged form of 18F-CPFPX in the plasma decreased rapidly <25% after 10 min after injection, whereas the percentages of unchanged 11C-MPDX in the plasma remained high during the 60-min PET scan: 89% at 10 min and 75% at 60 min after injection. Although 11C-MPDX (inhibition constant [Ki] = 4.2 nmol/L for the rat forebrain membrane) (10) has a slightly lower affinity for A1 receptors than 18F-CPFPX (Ki = 1.26 nmol/L for the cloned human A1 receptors) (25), the in vivo stability of 11C-MPDX is the advantage of the continuous input function for the kinetic analysis. On the other hand, 18F-CPFPX has practical advantages: 18F provides a slightly better resolution of the images and its longer half-life of is more suitable to handle in clinical use compared with 11C-labeled tracers.
Coexpression of and functional interactions between adenosine A1 and dopamine D1 receptors in the striatum of rat and rabbit have been reported (26). The similarity in distribution of these receptors types suggests that such an interaction might also occur in the human brain. They are important for cholinergic neurotransmission. Adenosine plays an important role in sleep, and adenosine receptor antagonists such as caffeine promote wakefulness and disrupt normal sleep (4,27). Studies on the postmortem human brain reported a reduced density of adenosine A1 receptors in the hippocampus of patients with Alzheimer’s disease (28–30). The anticonvulsant effects of adenosine appear to be mediated primarily by A1 receptors (31,32). Carter et al. reported that arousal detected by electroencephalography after caffeine ingestion might be due to increased cholinergic activity (33). Angelatou et al. detected a significant increase in adenosine A1 receptor binding in the neocortex obtained from patients with temporal lobe epilepsy (34), whereas Glass et al. found that the adenosine A1 receptors were reduced in epileptic temporal cortex in temporal lobes removed from patients with complex partial seizures (35). The 11C-MPDX PET is of great interest in establishing the diagnosis of patients with somnipathy, epilepsy, Alzheimer’s disease, and other neurologic and psychiatric diseases and understanding the pathogenesis and treatment effect.
CONCLUSION
11C-MPDX was widely but discretely distributed with different concentrations in the brain. The binding of 11C-MPDX was high in the striatum and thalamus, intermediate in the cerebral cortices, and low in the cerebellum. The distribution pattern of 11C-MPDX was consistent with that of adenosine A1 receptors in vitro but discretely different from that of rCBF. The 11C-MPDX PET has the potential for mapping adenosine A1 receptors in the human brain. 11C-MPDX PET is useful for mapping adenosine A1 receptors in the human brain.
Footnotes
Received Mar. 18, 2004; revision accepted Aug. 12, 2004.
For correspondence or reprints contact: Nobuyoshi Fukumitsu, MD, Proton Medical Research Center, University of Tsukuba, 1-1-1, Tennoudai, Ibaragi, 305-8575, Japan.
E-mail: GZL13162{at}nifty.ne.jp