Abstract
2′-Deoxy-2′-18F-fluoro-5-fluoro-1-β-d-arabinofuranosyluracil (18F-FFAU) has been synthesized and evaluated in HT-29 cells as a potential PET agent for herpes simplex virus type 1 thymidine kinase (HSV1-tk) gene expression. Methods: 2-Deoxy-2-18F-fluoro-1,3,5-tri-O-benzoyl-α-d-arabinofuranose was prepared by the reaction of the respective 2-triflate with tetrabutylammonium 18F-fluoride. The fluorosugar was converted to its 1-bromo derivative and coupled with protected 5-fluorouracil. The crude product was hydrolyzed in base and purified by high-performance liquid chromatography to obtain the 18F-FFAU. In vitro studies were performed in HT-29 cells by incubation at various time points. In vivo studies including biodistribution and microPET were performed in tumor-bearing nude mice. Results: The radiochemical yield was 20%–30% decay corrected with an average of 25% in 4 runs. Radiochemical purity was >99% and average specific activity was 85 GBq/μmol (2,300 mCi/μmol) (end of synthesis). In vitro accumulation of 3H-FFAU in HSV1-tk–expressing cells was ∼180-fold (P < 0.001) higher than that in the wild-type cells between 30 and 120 min. In vivo uptake of 3H-FFAU in HSV1-tk–positive tumors at 2 h was ∼8-fold (P < 0.001) higher than that in the control tumors. Tumor uptake (percentage injected dose per gram of tissue) and the uptake ratio (tk-positive to wild type) of 3H-FFAU in tk-positive cells was higher compared with those of our earlier studies using 2′-14C-deoxy-2′-fluoro-5-methyl-1-β-d-arabinofuranosyluracil (14C-FMAU) and 9-(4-18F-fluoro-3-hydroxymethylbutyl)guanine (18F-FHBG) in the same cell lines. microPET on tumor-bearing nude mice also demonstrated a very high uptake of 18F-FFAU in tk-positive tumors compared with that of the control tumor without significant accumulation in other organs. Conclusion: These results demonstrate that 18F-FFAU has superior biodistribution characteristics and significantly higher in vivo uptake in HSV1-tk–expressing tumor compared with previously studied agents.
- 2′-deoxy-2′-18F-fluoro-5-fluoro-1-β-d-arabinofuranosyluracil
- herpes simplex virus type-1 thymidine kinase
- HT-29 cells
- PET
- gene expression
Herpes simplex virus type-1 thymidine kinase (HSV1-tk) is being used as a suicide gene for gene therapy of cancer (1–9). In animal models malignant tumors have been successfully treated with suicide gene therapy using HSV1-tk gene and ganciclovir (6,7). However, clinical results with this method showed that gene delivery to the tumor cell in humans was not sufficient (8,9) for therapy. An in vivo method to assess the HSV1-tk enzyme activity after gene transfer is required to monitor gene expression as an indicator of gene delivery. Imaging of the HSV1-tk reporter gene along with various reporter probes is of current interest (2,10–12). Reporter genes can be used to assess vector targeting, the level of suicide gene (HSV1-tk) expression, and quantitatively monitor the level of enzyme in gene therapy with the help of in vivo imaging probes (2,3,5,13). Nuclear medicine, such as PET, can provide repeated, noninvasive, and quantitative assessment of the expression of genes in tissues and organs (2,4,10,13–15).
In contrast to the mammalian kinase, which phosphorylates thymidine preferentially, HSV1-tk can phosphorylate a wide range of nucleoside analogs such as acycloguanosines and 2′-deoxyfuranosyluracil nucleoside derivatives that are not phosphorylated efficiently by the native enzyme (1,11,12,15–18). The presence of fluorine in the 2′-arabino position in a furanosyluracil nucleoside results in enhanced monophosphorylation by HSV-tk type 1 and type 2 compared with host thymidine kinase (19).
We originally developed 9-(3-18F-fluoro-1-hydroxy-2-propoxymethyl)guanine (18F-FHPG) and 9-(4-18F-fluoro-3-hydroxymethylbutyl)guanine (18F-FHBG) for PET of HSV1-tk gene expression (11,12,20–22) and demonstrated that 18F-FHBG is more useful than 18F-FHPG for this purpose (21,22). Acyclovir (FACV), ganciclovir (FGCV), and pencyclovir (FPCV) labeled with 18F at the C-8 position have also been reported by others (16,17,23,24), with FPCV being a better reporter probe compared with FGCV (17).
Compared with the acyclonucleoside analogs, 18F-FHPG and 18F-FHBG, pyrimidine nucleoside derivatives 2′-deoxy-2′-fluoro-5-iodo-1-β-d-arabinofuranosyluracil (FIAU) and 2′-deoxy-2′-fluoro-5-methyl-1-β-d-arabinofuranosyluracil (FMAU) have been found to be much more sensitive probes (i.e., higher cellular accumulation) for PET of HSV1-tk gene (25,26). Among these probes, radioiodinated FIAU has been shown to be superior to the acycloguanosine derivatives FHPG and FHBG in some cell lines (26) in terms of total uptake and the uptake ratio (tk-positive to wild type), although it is susceptible to deiodination in vivo. Within the deoxyuridine series, the presence of fluorine in the 5-position in addition to the fluorine at the 2′-arabino position further enhances the antiviral activity of the respective compound against HSV (19). This suggested that 2′-deoxy-2′-fluoro-5-fluoro-1-β-d-arabinofuranosyluracil (FFAU) labeled with an appropriate isotope may posses an advantage over FIAU and other 5-substituted analogs for imaging gene expression. To identify an optimal PET agent for HSV1-tk gene expression, we have synthesized 18F-FFAU and studied it as a potential marker for this purpose. The results are compared with those from our earlier studies with 14C-FMAU and 18F-FHBG in HT-29 human colon carcinoma cells.
Our original synthetic procedure for radiolabeling FFAU using 18F-F2 produced 5-18F-FFAU in low specific activity (27). The more recent development of an efficient, reproducible synthesis for 18F-labeled pyrimidine nucleoside analogs with radiolabel in the sugar moiety (28,29) has afforded an opportunity to study these compounds for in vivo imaging of gene expression in animal models and humans.
MATERIALS AND METHODS
Radiotracers
3H-FFAU, with a specific activity of 444 GBq/mmol (12 Ci/mmol) and a radiochemical purity of >99%, was purchased from Moravek Biochemicals, Inc.
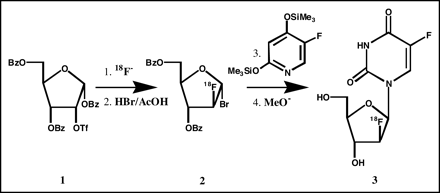
18F-FFAU was synthesized following a method developed in our laboratory (28,29) as shown in the synthetic scheme (Fig. 1). Briefly, 2-deoxy-2-trifluoromethanesulfonyl-1,3,5-tri-O-benzoyl-α-d-ribofuranose 1 was reacted with tetrabutylammonium 18F-fluoride to produce 2-18F-fluoro-1,3,5-tri-O-benzoyl-α-d-arabinofuranose, which was converted to its 1-bromo derivative 2 by treatment with hydrogen bromide in acetic acid (HBr/AcOH). The 18F-labeled bromosugar 2 was coupled with the protected 5-fluorouracil, and the coupled product was hydrolyzed in base. The crude product was purified by high-performance liquid chromatography (HPLC) to obtain the desired product 18F-FFAU 3.
Synthetic scheme for 18F-FFAU.
Cell Line and Transduction
Human colon cancer cells HT-29 were obtained from American Type Culture Collection, transduced with the retroviral vector G1Tk1SvNa following literature methods (30,31), and used as in earlier studies (21,22,25). The transduced cells were periodically tested for HSV1-tk expression and found to be stable during the studies. Nontransduced (wild type) cells were used as controls. The in vitro doubling time of transduced cells was 48 h and that of the wild-type cells was 24 h.
In Vitro Studies.
Cells transduced with retroviral vector carrying HSV1-tk gene and wild-type cells (1–2 million) were plated in duplicate to a Petri dish (100 mm) and cultured in 10 mL of media for 24 h. Logarithmically growing cells were incubated with ∼37 kBq (1 μCi) of 3H-FFAU for 30, 60, and 120 min. After incubation and removal of media, the cells were washed with ice-cold phosphate-buffered saline (PBS, 3 × 10 mL) and trypsinized. After neutralization with 10% fetal bovine serum in Dulbecco’s modified Eagle medium (1.5 mL), cells were centrifuged for 5 min at 1,000 rpm and supernatants were discarded. Cells were resuspended in cold PBS, and an aliquot was removed for cell counting. The remaining cells were centrifuged and the cell pellets were treated with 0.3N perchloric acid (PCA, 2 mL). The acidic mixtures were cooled to 4°C for 10 min and centrifuged for 1 min at 14,000 rpm, and the supernatant was separated. The cell pellets were washed twice with PCA (1 mL) and centrifuged, and the acid- soluble fractions, washings, and cell pellets were treated with complete counting scintillation cocktail (RIA-SOLVE-II; Research Products International Corp.). Radioactivity in the acid-soluble fractions, washings, and cell pellets were counted in a liquid scintillation counter (Beckman LS 9000) using the external standard method of quench correction. The number of cells was counted using a hemocytometer in the fractions of cell suspension removed from each experiment. The combined radioactivity in acid-soluble fractions, washings, and cell pellets was normalized to activity (dpm) per million cells, and an average was calculated with SD. Activity in the cell pellets was considered as uptake in macromolecules. The ratio of activity uptake between transduced and wild-type cells was obtained for each experiment per time point, and an average ratio was calculated with SD. To determine the molecular species distribution of the radiolabel, the acid-soluble fraction was neutralized with 2.5N KOH and analyzed by HPLC (25) to identify soluble nucleosides and nucleotides. The acid- insoluble fraction (cell pellet) was further analyzed for radioactivity in macromolecules as follows: The cell pellet was treated with KOH and heated overnight. The basic cell suspension was centrifuged and the supernatant was separated. The radioactivity in the cell pellet was considered as uptake in DNA, and activity in the supernatant was considered as uptake in RNA or in the FFAU-monophosphate-thymidine synthetase-H4-folate (FFAU-TS-H4-folate) ternary complex as reported earlier (32).
In Vivo Studies.
In vivo studies, including biodistribution, were conducted on tumor-bearing nude mice at 2 h after injection. Tumors were grown in 6- wk-old athymic nude mice (Harlan) by inoculation of ∼7 million cells (wild type) under the skin in the left thigh and ∼9 million cells (HSV1-tk positive) in the right thigh. When the tumor was about 1 cm in size, animals were used for the experiment as described under an approved Institutional Animal Care and Use Committee protocol.
One group of mice (n = 6) with tumors on each flank (wild in the left and transduced on the right) was injected intravenously via a tail vein with 3H-FFAU (∼37 kBq, 1 μCi, 200 μL). Blood samples were collected from tumor-bearing mice (n = 3) in capillary tubes (1–2 μL) from the contralateral tail vein after rupture with a needle. These samples were obtained at 2, 5, 10, 20, 60, and 120 min after injection. Blood samples were mixed with scintillation cocktail, activity in each sample was measured with the scintillation counter, and the percentage injected dose per gram (%ID/g) was calculated. Animals were anesthetized with sodium pentobarbital (∼40 mg/kg) and sacrificed at 2 h after injection. Organs and tumors were excised and weighed. Tissue samples were homogenized by a sonicator (BRAUN-SONIC-1510) and mixed with the scintillation cocktail. Activity was measured on the scintillation counter using the external standard method of quench correction. For each mouse, radioactivity uptake was expressed as the %ID/g of tissue and the ratio of tumor (tk-positive) to organ. Mean activity uptake was calculated with SD and compared between the control and experimental tumors. Statistical significance of comparison between the control and experimental tumors was based on a 2-tailed t test.
For PET, animals (n = 2) were injected with 18F-FFAU (∼7.4 MBq, 0.2 mCi) through the tail vein, and imaging were performed using a microPET scanner (Concorde Microsystems, Inc.; spatial resolution of 1.2 mm) at 30 min, 1 h, and 2 h after injection. Initial 30-min dynamic scans were performed to determine the kinetics for tumor uptake of the compound. Static scans were performed at 1 and 2 h with 10-min acquisitions. Images were reconstructed using an ordered-subset expectation maximization algorithm. Regional tumor radioactivity concentrations (kBq/cm3) were estimated from the maximum pixels within regions of interest drawn around the tumor on transaxial slices of the reconstructed image sets. The radioactivity uptake in tumor (kBq/cm3, μCi/cm3) was converted to %ID/g and compared with the biodistribution data using 3H-FFAU.
RESULTS
The radiochemical yield of 18F-FFAU was 20%–30% decay corrected with an average of 25% in 4 runs. Radiochemical purity was >99% and the average specific activity was 85 GBq/μmol (2,300 mCi/μmol) at the end of synthesis. The synthesis time was 3.5–4.0 h from the end of bombardment.
Figure 2 summarizes in vitro accumulation of 3H-FFAU in HSV1-tk–expressing and in control cells. Between 30 and 120 min, 3H-FFAU uptake in transduced cells was 176- to 81-fold (P < 0.001) higher than that in the wild-type cells (Fig. 2). Uptake in wild-type cells was too low compared with that in the transduced cells to visualize in the graph. HPLC analysis of the acid-soluble fraction showed one peak corresponding to FFAU (data not shown).
Incorporation of 3H-FFAU in HT-29 cells: wild type (short columns) and transduced with HSV-tk (tall columns).
Figure 3 represents incorporation of 3H-FFAU in macromolecules (ternary complex with TS) of wild-type and transduced cells at 30, 60, and 120 min. Approximately 80% of total intracellular activity was incorporated in the macromolecular fraction in the wild-type cells at all 3 time points, whereas 29%, 38%, and 42% was seen in the macromolecular fraction in HSV1-tk cells at 30, 60, and 120 min, respectively. Thus, although the data show that the total macromolecular uptake in transduced cells was 56- to 58-fold (P < 0.001) higher than that in the wild-type cells, the intracellular distribution was different between the 2 cell lines.
Incorporation of 3H-FFAU as ternary complex (macromolecule) in HT-29 cells: wild type (short columns) and transduced with HSV-tk (tall columns).
The blood clearance curve for 3H-FFAU in nude mice (n = 3) is shown in Figure 4. The peak radioactivity seen at 2 min was ∼40 %ID/g after tail vein injection, after which a rapid decrease was observed, followed by a very slow clearance after 20 min. The plasma half-life of the tracer in nude mice was calculated from the clearance curve to be ∼10 min. Approximately 7 %ID/g remained in the blood at 1 h, which subsequently cleared very slowly.
Blood clearance of 3H-FFAU in tumor-bearing nude mice.
Table 1 summarizes the biodistribution of 3H-FFAU in tumor-bearing nude mice at 2 h after injection. Uptake of 3H-FFAU in transduced tumors was 30.75 ± 7.43 %ID/g. Activity in blood and other organs was comparable, except slightly higher uptake in kidney. The uptake ratio between transduced and wild-type tumors was 7.9 (P < 0.001). The ratio between tk-positive tumor and blood at this time was >10, and that between control tumor and blood was 1.3.
Biodistribution of 3H-FFAU in Tumor-Bearing Nude Mice at 2 Hours After Injection
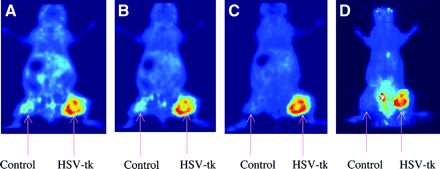
microPET images of tumor-bearing mice using 18F-FFAU are shown in Figure 5: (A) 30-min coronal image; (B) 60-min coronal image; (C) 120-min coronal image; (D) 120-min projection image. Tumors were grown with HT-29 cells: wild-type cells on the left flank and transduced cells on the right flank. As the images show, 18F-FFAU accumulates only in tk-positive tumors on the right flank, consistent with the biodistribution results. All other organs demonstrated low accumulation at 2 h except the bladder. Uptake in wild-type tumor is as low as the background activity by 2 h. Some apparent background activity in the projection image taken at 2 h (Fig. 5D) as opposed to the single frame at the same time point (Fig. 5C) is due to urine on the scanner bed. The hottest spot is the bladder, followed by the transduced tumor on the right flank.
microPET images of 18F-FFAU in tumor-bearing nude mice.
Figure 6 represents tumor uptake in the first 30 min from dynamic PET scans. Uptake in tk-positive tumor rises in the first 5–10 min and subsequently plateaus, consistent with the in vitro data. This result demonstrates very high in vivo trapping of 18F-FFAU in HSV-tk–positive cells compared with that in the wild-type cells.
Uptake of 18F-FFAU in tumors from dynamic PET scans.
A comparison between 3H-FFAU and results from our earlier studies with 14C-FMAU and 18F-FHBG in this transduced tumor model is represented in Figure 7. The tumor uptake of FFAU, FMAU, and FHBG was 30.75 ± 7.43, 11.35 ± 2.75, and 0.30 ± 0.03 %ID/g, respectively. Uptake of FFAU was ∼3-fold higher than that of FMAU and ∼100-fold higher than that of FHBG in tk-positive tumors. The uptake ratio between transduced and nontransduced tumors for FMAU and FHBG was comparable, whereas that of FFAU was much higher.
Comparison of tumor uptake in HSV-tk–expressing cells between FFAU, FMAU, and FHBG.
DISCUSSION
The objective of this work was to develop a radiotracer suitable for in vivo imaging of HSV1-tk expression using PET. FFAU was chosen on the basis that the additional fluorine at the 5-position enhances the antiviral activity above and beyond that due to the fluorine in the 2′-arabino position against HSV type 1 and type 2 (19). The in vitro and in vivo experiments were designed to provide a comparison between FFAU and other tracers under investigation as gene imaging agents—namely, FMAU and FHBG. The results presented herein suggest that FFAU is a substrate for HSV1-tk enzyme and may have superior imaging characteristics compared with other tracers such as FMAU and FHBG for assessment of gene expression with PET.
In vitro studies revealed that the total accumulation of 3H-FFAU in HSV1-tk–expressing cells was significantly higher than that in wild-type control cells and increased rapidly in the transduced cells, reaching a plateau by 60 min (Fig. 2). Maximum incorporation of FFAU within a short time (30 min) suggests that the rate of monophosphorylation by the HSV1-tk was also very high. This is further supported by the dynamic PET scans, which showed that tumor uptake peaks within 10 min of injection and remains elevated throughout the time course studied (Fig. 6). The in vitro uptake ratios between transduced and wild-type cells were 176, 126, and 81 at 30, 60, and 120 min, respectively. There was a gradual but slow accumulation of FFAU in wild-type cells likely representing minimal baseline monophosphorylation by host kinase and irreversible binding to TS as previously suggested (32) throughout the 2 h study period. No significant increase in uptake in transduced cells was observed after 60 min, however. As a result, the ratio between tk-positive and wild-type cells decreased from 176 to 81 by 2 h.
HPLC analysis of the acid-soluble fraction from transduced cells showed only the parent compound 3H-FFAU (data not shown), consistent with the fact that there was no free monophosphate and no indication for formation of di- or triphosphates. Activity in the acid-soluble fraction from wild-type cells was too low to detect by HPLC due to the overall low total cellular uptake in these cells compared with that in transduced cells. Basic hydrolysis of the acid-insoluble fractions from both cell lines after centrifugation provided 2 fractions, the cell pellet and the supernatant. The cell pellet containing the DNA and the supernatant containing the RNA and any macromolecular complexes, including TS, were analyzed. In both cell lines, 3H-FFAU was not found in the DNA fraction as opposed to our earlier results with 14C-FMAU (25) All remaining macromolecular activity was contained in the supernatant, presumably either in RNA or in another form such as a ternary complex with TS (32). HPLC analysis of the supernatant from the basic hydrolysis of acid-insoluble fractions from the transduced cells showed 17% FFAUMP and 83% free FFAU, presumably from the dissociation of the TS complex; the supernatant from wild-type cells, however, was too low for accurate HPLC analysis. Coderre et al. (32) demonstrated earlier that FFAUMP forms a stable complex with TS and methylene tetrahydrofolate in a manner analogous to that for 5-fluorodeoxyuridine monophosphate, which could be isolated and hydrolyzed by a base. Our observation is consistent with the earlier report that FFAUMP forms a stable complex. The quasiirreversible formation of the complex of FFAUMP with TS prohibits the formation of FFAU di- and triphosphates and, hence, its incorporation into DNA. Although wild-type cells could not be evaluated using the methods described because of the very low overall cellular uptake, the absence of monophosphate in the acid-soluble fraction from the transduced cells suggests that HSV-tk cannot efficiently phosphorylate FFAU. The higher level of total cellular uptake—that is, trapping—in transduced cells therefore may be a result of formation of a complex between FFAU and HSV-tk, which does not survive the acid hydrolysis, leading to high levels of the free compound on analysis. Likewise, a plateau effect could occur as binding sites on HSV-tk enzyme are consumed. The lower percentage of macromolecular accumulation in transduced cells compared with that in wild-type cells also suggests that there may be competition between TS and HSV-tk for FFAU.
For in vivo studies, tumors were grown by injection of different amounts of cells (∼7 million for wild-type and ∼9 million for transduced) due to differences in doubling time of these lines. The doubling time of wild-type cells is ∼2 times that for the transduced cells. To maintain similar tumor volumes, fewer wild-type cells were injected in the animals. Despite these efforts, the wild-type tumor grew marginally larger over the same period of time before use. Data were normalized to %ID/g in an effort to reduce the effects of tumor size variations.
In vivo results also revealed that tumor uptake in transduced cells is much higher than that in nontransduced cells (Table 1). Uptake of 3H-FFAU in transduced and wild-type tumors was 30.75 ± 7.43 and 3.87 ± 1.80 %ID/g, respectively. Uptake in other organs, including blood, was quite low, except kidney, which was slightly higher with renal clearance being the primary route of radiotracer excretion. The uptake ratio between transduced and wild-type tumor was 7.9, whereas the transduced tumor-to-blood ratio was 10.4. As with the cell studies, the higher uptake in transduced tumor indicates accumulation of FFAU, presumably due to reversible binding to or complex formation with HSV-tk with net intracellular substrate trapping. As opposed to FMAU (25), no significant uptake in wild-type tumor was observed, suggesting that FFAU undergoes only minimal phosphorylation by the host kinase over the 2 h period studied.
microPET images on tumor-bearing mice using 18F-FFAU (Fig. 5) also show that 18F-FFAU accumulates significantly only in tk-positive tumors, consistent with the biodistribution results. All other organs demonstrate low uptake at 2 h except bladder. Uptake in wild-type tumor was as low as the background activity. These results from biodistribution and microPET images suggest that 18F-FFAU may be useful for localization of undesirable HSV-tk expression in nontarget tissue in future safety trials.
To compare the efficacy of these compounds, FFAU, FMAU, and FHBG in this cell line (HT-29), uptake in transduced tumors, and the ratios of tumor uptake in transduced versus nontransduced cells were calculated and plotted (Fig. 7). Figure 7 shows that total uptake of 3H-FFAU in vivo (%ID/g) was much higher compared with that of FMAU and FHBG, and the uptake ratio between transduced and wild-type cells at 2 h for FFAU was also higher than that of FMAU and FHBG, whereas the ratio was comparable between FHBG and FMAU. Although the metabolic pathway of FFAU is different from that of FMAU, FHBG, and FIAU, the high accumulation (sensitivity) and the uptake ratio (specificity) of FFAU in tk-positive cells, along with its in vivo stability and desired biodistribution characteristics, suggest it is superior to other agents studied to date in this HT-29 cell line.
Finally, our recent synthesis of 18F-FFAU produced a very high specific activity product in reasonable yield, which is expected to have an advantage over the earlier synthesis using 18F-F2 (27). The current synthesis using 18F-fluoride requires a longer time compared with the other synthesis with 18F-F2 but is more efficient and reproducible and provides sufficient quantities of material for animal and human imaging studies.
CONCLUSION
In vitro and in vivo studies indicated that radiolabeled FFAU preferentially accumulates to a much higher degree in transduced HT-29 cells compared with that in the wild-type cells. In comparison with FMAU and FHBG, the sensitivity of FFAU was 3-fold and ∼100-fold higher than that of FMAU and FHBG, respectively, with an uptake ratio higher than that of both FMAU and FHBG. These results along with the low accumulation observed in normal organ and tissues suggest that 18F-FFAU may be a useful PET agent for HSV1-tk gene expression and superior to 18F-FMAU and 18F-FHBG. The mechanism(s) of intracellular trapping of FFAU requires further exploration.
Acknowledgments
This work was supported in part by National Cancer Institute grants CA 72896 and P20 CA 86532.
Footnotes
Received Jun. 28, 2004; revision accepted Sep. 10, 2004.
For correspondence or reprints contact: Peter S. Conti, MD, PhD, 2250 Alcazar St., Suite 101, Los Angeles, CA 90033.
E-mail: pconti{at}hsc.usc.edu