Abstract
β-Adrenergic blocking agents are widely used in coronary artery disease (CAD), although their impact on myocardial blood flow (MBF) and coronary flow reserve (CFR) remains unclear. We studied the effect of long-term β-blocker treatment (carvedilol or metoprolol) on coronary microcirculation in CAD patients using PET. Methods: Regional and global resting and adenosine-induced hyperemic MBF and CFR were measured with 13N-ammonia and PET in 36 CAD patients before and after 12 wk of oral therapy with either carvedilol, 50 mg/d, or metoprolol, 100 mg/d. Results: β-Blockade decreased global resting MBF in proportion to cardiac work (from 0.86 ± 0.20 to 0.77 ± 0.14 mL/min/g, P < 0.05) without affecting global hyperemic flow. Hyperemic MBF was significantly lower in stenosis-dependent segments than in remote segments (1.76 ± 0.64 vs. 2.04 ± 0.67 mL/min/g, P < 0.05) at baseline but was comparable in both after treatment (2.02 ± 0.68 vs. 1.90 ± 0.78 mL/min/g, P = not statistically significant [NS]), resulting in a significant CFR increase in stenotic segments (+15%, P < 0.05) but not in remote segments (+9%, P = NS). Conclusion: The beneficial effect of β-adrenergic blockade can be explained by the reduction in oxygen consumption (= decreased demand) but also by a modest improvement in vasodilator capacity (= increased supply). The improvement in CFR is found predominantly in stenosis-dependent rather than remote segments.
In patients with coronary artery disease (CAD), coronary flow is impaired during hyperemia (exercise stress or adenosine) (1). This impairment is found even in coronary segments with nonsignificant or no stenoses at all (2).
β-Receptor blockers reduce myocardial oxygen consumption and myocardial blood flow (MBF) by reducing myocardial contractility and extravascular resistive forces. The latter have been demonstrated to impede coronary blood flow during pharmacologic vasodilation (3). Therefore, β-receptor blockade might also alter MBF during near-maximal coronary vasodilation. In fact, in healthy volunteers, short-term β1-receptor blockade reduces resting MBF and increases pharmacologic hyperemia, resulting in an increased coronary flow reserve (CFR) (4). However, the influence of β-blockade on MBF has not yet been explored in depth in patients with CAD.
The aim of the present study was, therefore, to quantify noninvasively with PET the effect of β-receptor blocker treatment on MBF and coronary vasodilatory capacity in patients with CAD. A secondary endpoint was the comparison of metoprolol (a selective β1-receptor–blocking drug) and carvedilol (a nonselective β-blocking drug with vasodilating properties mediated by α1-receptor inhibition and antioxidant effects (5)).
MATERIALS AND METHODS
The study protocol was approved by the local ethics committee. All subjects gave informed and written consent before the study.
Study Population
The study population consisted of 36 patients (mean age of 59 ± 7 y) with angiographically documented CAD, stable angina pectoris, and at least 2 cardiovascular risk factors. All patients had subjective or objective signs of ischemia during exercise stress. CAD was defined as one or more coronary arteries whose diameter was found to be narrowed by >50% at the time of primary diagnosis. Daily visual assessment of diameter stenosis was routine clinical practice at our institution. Patients who had undergone coronary angioplasty >6 mo previously were included if they had at least one residual coronary artery with diameter narrowing of >50% after angioplasty. Patients who had undergone coronary angioplasty within the previous 6 mo or had an abnormal ejection fraction were excluded from the present study. Thus, ejection fraction was normal in all patients, although 15 patients had experienced a prior minor myocardial infarction. Infarcted segments were excluded from the analysis. After randomization of patients to either metoprolol or carvedilol treatment, we found that an average of 2.4 and 2.3 vessels was affected in the metoprolol group and the carvedilol group, respectively.
Study Design
Any antiischemic therapy was withdrawn for 1 wk, during which only short-term nitrates were allowed, and the baseline PET study followed. All volunteers were carefully instructed to refrain from caffeine intake for 24 h before the PET study. Patients were then randomly assigned to receive either oral metoprolol or carvedilol treatment. In both groups, the β-blocker dosage was titrated over 1 wk to the maximal dose of 100 mg of metoprolol per day or 50 mg of carvedilol per day. Because carvedilol is administered at 25 mg twice daily whereas metoprolol requires only a single dose per day, the metoprolol group received a visually identical additional placebo to maintain the double-blinded design of the study. The placebo tablet was provided by Roche Pharma (Schweiz) AG. After 12 wk of treatment, all patients underwent a second PET study. Thereafter, the initial treatment was reinstated.
PET
Scanning was performed in the PET Center of the University Hospital Zurich on an Advance PET tomograph (General Electric Medical Systems; axial field of view, 35 × 4.25 mm). A 30-min blank scan was recorded as part of the daily routine procedure. The optimal imaging position was determined by a 2-min rectilinear scan after exposure of an external 68Ge ring source. All volunteers received a 700- to 900-MBq injection of 13N-ammonia into a peripheral vein before acquisition of heart images began (nine 10-s, six 15-s, three 20-s, two 30-s, and one 900-s frames). After a 20-min acquisition, enough time was allowed for decay of 13N-ammonia, and a 20-min transmission scan for photon attenuation correction was then obtained using external 68Ge sources. MBF was assessed at rest and during standard pharmacologic stress, that is, a 7-min infusion of adenosine, 0.14 mg/min/kg of body weight (6).
Data Analysis
Images were transferred to a personal computer (model 2200; Transtec AG) and analyzed with the PMOD software package (PMOD Technologies Ltd.). Regions of interest (ROIs) were drawn semiautomatically using a centerline within the myocardium in the short-axis projection. The junctions of the right and left ventricles were marked to indicate the septum. The left ventricular free wall was then subdivided geometrically into 3 segments of the same size. According to the recommendations of the American Society of Echocardiography (7), the left ventricle was subdivided into a total of 16 segments. An ROI was placed in each segment as well as into the left and right ventricular blood pools. Segments with myocardial infarction were excluded from the present analysis. All remaining segments were categorized as either stenosis dependent or remote by 2 independent interpreters of the coronary angiography findings who were unaware of the MBF values. Agreement was 90%; in cases of disagreement, the segment was assigned to a category by consensus. In addition, for each patient the area subtended by the most severe stenosis (i.e., the area of putative ischemia) and a remote area subtended by an angiographically normal artery or an artery with minimal disease (i.e., a control area) were identified.
MBF was estimated by model fitting of the blood pool and myocardial time–activity curves (8). Correction for partial volume and spillover (both accounting for the resolution distortion) was performed as previously reported (9–11) using the method developed (12) and validated (13) by Hutchins et al. Briefly, the ROI is chosen to contain only myocardial tissue and blood; thus, the relationship between the measured PET counts in a region (CPET) and the true counts in myocardium (Cm) and arterial blood (Ca) is modeled as follows: CPET(t) = FaCa(t) + (1 − Fa)Ca(t). Fa is the fractional contribution of the blood pool to measured PET counts in a region and is dependent on the placement of the region, the resolution of the camera, and movement of the myocardium. Because the contribution of myocardium to total regional counts decreases with increasing blood-pool fraction, Cm is multiplied by (1 − Fa). Fa is estimated together with the other kinetic tissue parameters using least-squares fitting. Determination of Fa by either the measurement or the parameter estimation procedure eliminates the resolution distortion in the kinetic data. This strategy of spillover correction seems to be the most appropriate (9) in view of the potential heterogeneity in myocardial wall thickness in patients with CAD (13). CFR was calculated as the ratio of hyperemic to resting MBF values.
Blood pressure was continuously monitored by an upper-arm cuff (model 9300 blood pressure monitor; CAS Medical Systems Inc.) and recorded every minute. The electrocardiogram (ECG) was monitored continuously throughout the procedure, and a 12-lead ECG was recorded at baseline and every minute during adenosine administration and during 3 min of recovery.
Statistical Analysis
Hemodynamic data, MBF, and CFR were compared between the different study conditions with ANOVA statistics for repeated measures. If the P value was below 0.05, the Scheffé test was applied. Data, including percentage differences, are reported as mean values ± SD.
RESULTS
All procedures were well tolerated, apart from the common side effects of adenosine. No subjects experienced relevant ECG changes during the procedure. In the metoprolol group, 3 patients were lost to analysis because of dropout and, mainly for technical reasons, 4 patients could not be completely analyzed (1 in the metoprolol group and 3 in the carvedilol group). The remaining 29 patients (15 in the carvedilol group and 14 in the metoprolol group) were included in the analysis.
Hemodynamics
The resting rate–pressure product (RPP) declined from 9,648 ± 2,151 at baseline to 7,790 ± 1,729 (P < 0.0001) during carvedilol treatment and from 9,437 ± 2,399 at baseline to 7,835 ± 1,329 (P < 0.005) during metoprolol treatment (no significant difference between the groups). The decrease in systolic blood pressure was slightly larger with carvedilol (−5%, P < 0.05) than with metoprolol (−3%, P = not statistically significant [NS]), possibly because of the peripheral α1-blocking effect of carvedilol (Table 1). Similarly, the adenosine-induced increase in RPP was slightly more attenuated by carvedilol (−20%, P < 0.0001) than by metoprolol (−10%, P < 0.05).
Hemodynamics
MBF and CFR
Resting MBF in remote segments decreased significantly after carvedilol (−9%, P < 0.05) and metoprolol (−11%, P < 0.05), with no significant difference between the 2 groups. By contrast, in stenotic segments a significant decrease in MBF was found after metoprolol (−10%, P < 0.05) but not after carvedilol (−1%, P = NS) (Fig. 1).
Treatment-induced changes in resting MBF. Resting MBF decreased in both treatment groups in remote segments. In stenotic segments, however, metoprolol decreased MBF (−10%, P < 0.05) whereas carvedilol did not (−1%, NS), improving the oxygen consumption/supply balance.
Hyperemic MBF overall and in both treatment groups was significantly lower in stenotic segments than in remote segments (1.76 ± 0.64 vs. 2.04 ± 0.67 mL/min/g, P < 0.05) at baseline but was comparable in both types of segments after treatment (2.02 ± 0.68 vs. 1.90 ± 0.78 mL/min/g, P = NS) (Fig. 2; Tables 2 and 3). Carvedilol showed a trend to increase hyperemic MBF in stenotic segments (from 1.65 ± 0.60 to 1.91 ± 0.85 mL/min/g), but the trend fell short of statistical significance. Metoprolol had no influence on hyperemic flow in stenotic segments (1.89 ± 0.68 vs. 1.87 ± 0.73 mL/min/g, P = NS).
Effect of treatment on hyperemic MBF. In both groups, hyperemic MBF was significantly lower in stenotic segments than in remote segments before treatment. This difference disappeared after treatment with either drug.
Individual Values for Myocardial Blood Flow
MBF and CFR
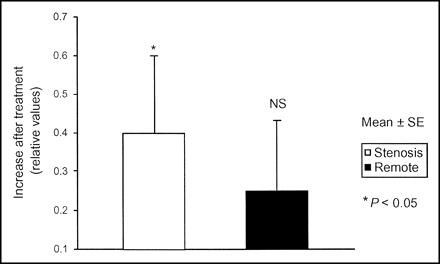
After treatment with either drug, CFR increased significantly in stenotic segments (+0.4 ± 0.2 [+15%], P < 0.05) but not in remote segments (+0.2 ± 0.2 [+9%], P = NS) (Fig. 3). This improvement was best seen in the most severe stenosis (Fig. 4). A trend toward an increased CFR in stenotic segments was more pronounced in the carvedilol group than in the metoprolol group (+18% vs. +12%, P = NS).
Treatment-induced changes in CFR. Pooled data of both groups showed that regional CFR increased significantly in stenosis-dependent segments, rather than in remote segments.
Relationship between lesion severity and CFR. CFR was lowest in the most severe stenosis but increased significantly after treatment.
DISCUSSION
The present study demonstrated that β-adrenergic receptor blockade modulates resting MBF and CFR in patients with CAD. This finding agrees with the findings of a previous study of healthy volunteers by Bottcher et al., who found a decrease in MBF and an increase in hyperemic flow after acute oral treatment with metoprolol (4). However, our long-term study of patients with CAD revealed, for what is to our knowledge the first time, a differential regional MBF response to β-adrenergic receptor blockade in CAD, that is, a increase in CFR that is more pronounced in ischemic than in control segments. Because of the consequent reduction in MBF heterogeneity between ischemic and control segments, the sensitivity of radionuclide perfusion scans for detection of CAD may be impaired, supporting the recommendation that patients should refrain from taking these drugs before diagnostic scanning.
Influence of β-Adrenergic Blockade on MBF at Rest
The beneficial effects of β-blocking agents have been attributed to the reduction in heart rate, the reduction in blood pressure, and the reduction in myocardial contractility (14,15), all of which reduce myocardial oxygen consumption. The reduction in MBF after intravenous administration of propranolol (16,17) and carvedilol (18) has been associated with a decrease in coronary luminal area (19–21) due to the unopposed α-adrenergic receptor vasomotor tone after blockade of the β-adrenergic receptors of the epicardial coronary arteries. In experimental animals, however, the decrease in coronary cross-sectional area after intravenous administration of propranolol and atenolol, a selective β1-adrenergic receptor blocker, was not prevented by α-adrenergic receptor blockade (22). This finding suggests that the effects are probably related mostly to the decrease in heart rate and contractility rather than to unopposed α-adrenergic receptor tone alone. Consistently, Gaglione et al. found no epicardial coronary vasoconstriction after intracoronary administration of 1 mg of propranolol (23).
In the present study, both carvedilol and metoprolol induced a significant decrease in cardiac work as reflected by the decrease in RPP. Both drugs decreased resting MBF to remote segments, but carvedilol, in contrast to metoprolol, did not decrease resting MBF to stenosis-dependent segments. This difference between carvedilol and metoprolol could be due to the additional vasodilatory effect of carvedilol, based on its α-adrenergic and antioxidant properties, and may infer an additional clinical benefit to carvedilol of further improving the oxygen demand–supply mismatch of stenotic segments. Such an effect may in part be involved in the mechanisms by which carvedilol treatment was able to stabilize or improve MBF and recruit viable segments in the recent CHRISTMAS trial (24). It may also have contributed to the superiority of carvedilol over metoprolol in the recent COMET trial (25), although our data provide no prognostic information to support such a hypothesis.
Influence of β-Adrenergic Blockade on MBF During Adenosine-Induced Hyperemia
In our study, a CFR increase after treatment was found predominantly in the segments with the most severe stenosis (Fig. 4) rather than in control segments. An increased CFR does not necessarily reflect an increased supply, as the increase in CFR was mainly due to a decrease in resting MBF. However, the concept of CFR allows appreciation of the available reserve (i.e., flow supply) that can be recruited to match an increase in flow demand. Similarly, Billinger et al. found an increase in coronary flow velocity reserve in CAD patients after administration of intravenous metoprolol due to decreased resting flow and increased hyperemic flow velocity (26). However, their results could not be extrapolated to the clinical setting of CAD for 2 reasons: First, measurements were performed in a successfully dilated vessel now free of obstructive disease; second, acute changes in coronary flow and changes in distending pressures of the distal vessel routinely affect coronary vasomotion and MBF after percutaneous transluminal angioplasty, as previously reported by their group (27) and others (28–30). These effects may have been interfering with their study protocol, rendering correct interpretation difficult.
In the present study, hyperemic MBF was significantly lower in stenotic segments than in remote segments before β-blockade—a finding that is in line with previous reports (31). After β-adrenergic blockade, however, there was no longer a difference because of the clear, although nonsignificant, trend toward an increased flow in stenosis-dependent segments (slightly stronger after carvedilol).
A reduction in extravascular contractive forces may have been involved. These extravascular resistive forces are determined by physical compression of intramural arteries and shear forces. They narrow coronary arteries as the heart contracts and impede MBF, particularly during pharmacologic vasodilatation (5). These forces can be modulated substantially by changes in left ventricular contractility. β-Adrenergic blockade may have affected diastolic relaxation, adding to decreased extravascular compressive forces and improved vasodilatory capacity. In addition, it cannot be ruled out that treatment may have modified stenosis diameter.
Study Limitations
Because atherosclerosis is a systemic disease and impairment in MBF is found even in coronary segments with nonsignificant or no stenosis, the potential existed for differences between stenotic and remote segments in our study to be underestimated (2). Therefore, we identified in each patient the segment subtended by the most severe stenosis and a remote area subtended by an angiographically normal artery or an artery with minimal disease, as given in Table 2.
In addition, all patients were on β-blocker treatment before inclusion in the study. Despite the 1-wk washout period, long-term effects from this treatment could have led to a further underestimation of our results. Thus, both apparent limitations seem to strengthen our results. Although the exact mechanisms of improved CFR after β-adrenergic receptor blocker cannot be elucidated, one can assume that changes in extravascular compressive forces may account for the improved vasodilatory capacity. These forces have a greater effect on endocrinal than epicardial layers, but the spatial resolution of the PET scanner did not allow such differentiation.
CONCLUSION
The beneficial effect of β-adrenergic blockade can be explained by the reduction in oxygen consumption (= decreased demand) but also by a modest improvement in vasodilator capacity (= increased supply). The latter is more pronounced with carvedilol than with metoprolol, possibly because of the additional α1-blocking and antioxidant properties of the former. The fact that CFR is improved predominantly in stenosis-dependent segments, rather than in remote segments, provides the pathophysiologic ground for withdrawal of β-adrenergic blockade before radionuclide perfusion scanning, as β-blockers decrease the contrast between ischemic and control areas during hyperemia.
Acknowledgments
We are grateful to Thomas Berthold, head radiographer, and his team for the excellent technical assistance and to Dr. Monika Hänggi and Dr. Anna-Katharina Küenzle for their support. This study was supported by a grant from the Swiss National Science Foundation (SNSF-Professorship grant PP0A-68835) and by a grant from Roche Pharma Schweiz AG.
Footnotes
Received Dec. 27, 2003; revision accepted Apr. 23, 2004.
For correspondence or reprints contact: Philipp A. Kaufmann, MD, University Hospital C NUK 32, Ramistrasse 100, CH-8091 Zurich, Switzerland.
E-mail: pak{at}usz.ch