Abstract
Indirect estimations of brain neurotransmitters in patients with anorexia nervosa (AN) and low weight have demonstrated a reduction in brain serotonin (5-HT) turnover in general and led to hypotheses about dysfunction in the 5-HT2a receptor system. It was our aim to investigate the central 5-HT2a receptor binding index using SPECT brain imaging. Methods: The 5-HT2a receptors of low-weight patients with AN were studied by means of the highly specific radioiodinated 5-HT2a receptor antagonist 4-amino-N-[1-[3-(4-fluorophenoxy)propyl]-4-methyl-4-piperidinyl]-5-iodo-2-methoxybenzamide or 123I-5-I-R91150. Fifteen patients with clinical diagnoses of AN and 11 age-matched healthy volunteers received intravenous injections of 185 MBq 123I-5-I-R91150 and were scanned with high-resolution brain SPECT. Results: Compared with healthy volunteers, patients with AN had a significantly reduced 5-HT2a binding index in the left frontal cortex, the left and right parietal cortex, and the left and right occipital cortex. A significant left-right asymmetry was noted in the frontal cortex (left < right). Conclusion: These results are in accordance with diminished metabolic and perfusion of frontal and parietal cortices reported in recent neuroimaging studies and imply localized disturbed serotonergic function. The data are discussed in the light of possible confounding factors related to the low-weight AN status. A regional cortical reduction in 5-HT2a binding index is not likely to be caused by a general reduction in serotonergic function due to the possible confounding factors. Suggestions for further research are given.
The essential features of anorexia nervosa (AN) are that the individual refuses to maintain a minimally normal body weight and is intensely afraid of gaining weight. Weight loss can be accomplished primarily through excessive physical exercise and through voluntary reduction in total food intake, sometimes accompanied by purging behavior (i.e., self-induced vomiting or the misuse of laxatives or diuretics). In addition, a disturbance in the perception of body shape and weight is an essential neuropsychologic feature of AN (1).
Psychologic and biologic mechanisms appear to play key roles in the pathogenesis of AN. Neuropsychologic investigations have indicated cognitive deficits in the frontal and parietal cortices (2,3). Perceived distortion of body image has been associated specifically with parietal dysfunction (4). Functional brain imaging with PET and SPECT has shown reduced parietal and frontal cortex metabolism (5) and cerebral perfusion (6).
In past decades, the involvement of serotonin as a neurotransmitter in AN was largely indicated through indirect estimations of brain serotonergic function. Impaired serotonin turnover and function were demonstrated through plasma measurements of tryptophan, the precursor of 5-hydroxytryptamine (5-HT) (7) or 5-hydroxyindole acetic acid (5-HIAA, the metabolite of 5-HT), in cerebrospinal fluid (CSF) of low-weight AN patients (8). Elevated CSF 5-HIAA concentrations have been reported in long-time weight-restored AN patients (8). Another indication of reduced serotonergic function in low-weight AN patients was blunted physiologic response to the administration of selective pharmacologic agonists (9–11). Again, these findings normalized after weight restoration (10).
The involvement of the 5-HT2a receptor in the pathophysiology of AN was demonstrated indirectly via blood platelet studies, both through an enhanced mobilization of intracellular platelet calcium content, mediated by 5-HT2 receptors (12), and through enhanced platelet serotonin 5-HT2a binding, measured in vitro with 3H-lysergic acid diethylamide (13). Recently, studies with PET and 18F-altanserin as a tracer showed reduced 5-HT2a binding in the orbitofrontal cortex in recovered bulimic patients (14). Some recent molecular genetic studies in patients with eating disorders have demonstrated an increased frequency of one of the alleles on the promotor region of the 5-HT2a gene (15). Also, twin and family studies suggest a genetic vulnerability to AN, and the hypothesis has been put forward that this vulnerability may be expressed in the central serotonergic system (16).
Functional imaging techniques, such as PET and SPECT using specific 5-HT2 receptor ligands, make it possible to evaluate in vivo receptor binding in patients with AN. Preliminary research in healthy subjects has indicated that 4-amino-N-[1-[3-(4-fluorophenoxy)propyl]-4-methyl-4-piperidinyl]-5-iodo-2-methoxybenzamide (123I-5-I-R91150) is a suitable ligand for imaging 5-HT2a receptors in vivo. It binds reversibly and with high affinity in vitro to 5-HT2a receptors (17). On average, 2% of a bolus dose of 123I-5-I-R91150 is taken up by the brain (18). Effective blockade of 5-HT2a receptors in vivo was demonstrated in a study of patients with schizophrenia who were treated with risperidone or clozapine (19).
The aim of this study was to evaluate the 5-HT2a binding index (BI) in patients with AN.
MATERIALS AND METHODS
Patients
Fifteen patients were included in the study. All were right handed, 16–30 y old, and diagnosed as having AN according to the criteria of the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (1). Patients were recruited from an inpatient eating-disorder unit in a university psychiatric hospital. Patients were selected by a senior psychiatrist and a senior psychologist after DSM-IV-based structured clinical interviews. To create a group of patients with consistent disease status, only symptom-fixed patients who stayed in the hospital as a result of resistance of the disease to treatment were included. Exclusion criteria were a comorbid psychiatric, major medical, or neurological disorder; antidepressant, neuroleptic, or electroconvulsive therapy in the preceding year; substance abuse; pregnancy or lactation; and a Mini-Mental State Examination score < 28. Menstrual status was noted in female patients.
The patients were compared with 11 age-matched healthy volunteers (7 women, 4 men) who were recruited from hospital staff. These individuals had no history of psychiatric treatment or significant medical events. None used psychotropic drugs or other relevant medication, and none abused illegal drugs. All had normal physical examination findings.
Ethical approval for the study was granted by the local ethics committee. Both patients and healthy individuals provided written informed consent to participate in this study.
Scanning Procedure
SPECT scanning was performed using 123I-5I-R91150 as a radioligand. This tracer was synthesized by electrophilic substitution on the 5-position of the methoxybenzamide group of R91150, followed by purification with high-performance liquid chromatography. The product had a radiochemical purity > 99% and was negative for bacteria and pyrogen tests. A specific activity of 370 GBq/μmol was obtained.
The tracer is a 5-HT2a antagonist with high affinity (Kd = 0.11 nmol) and selectivity for 5-HT2a receptors. The selectivity of the ligand for the 5-HT2a receptors is at least 50 times that of other receptors, such as other 5-HT receptors including 5-HT2c, dopamine receptors, adrenoreceptors, and histamine receptors (17).
Thyroid blockade was achieved by administration of a single oral dose of 100 mg potassium iodine before injection. All subjects received an intravenous injection of 185 MBq 123I-5-I-R91150 in normal sitting conditions. SPECT scanning was performed using a triple-head high-resolution gamma camera GCA-9300 (Toshiba Medical Systems, Oetwil am See, Switzerland) with fanbeam collimation. For 123I, the resulting transaxial image resolution is 9.5 mm in full width at half maximum.
Because sequential dynamic SPECT brain scans have shown that the corticocerebellar ratio reaches a plateau between 90 and 110 min, reflecting pseudoequilibrium, and remains stable thereafter for up to 8 h (18), acquisition was started between 110 and 140 min after tracer injection, according to previous protocols (20). A transmission scan was acquired before the emission scan, using 3 153Gd rod sources. This scan was used for subsequent image coregistration to stereotactic coordinates. Emission images were acquired during a 40-min period. The whole-brain volume was acquired within the single scanning session. Images were reconstructed using filtered backprojection and corrected for scatter and nonuniform attenuation (21).
Analysis of the scans was performed without knowledge of patient status after automatic image coregistration to stereotactic space using the transmission image (BRASS; Nuclear Diagnostics Ltd., Stockholm, Sweden) (22).
The method for coregistration of SPECT receptor data using transmission images has been published previously (22). In short, the sequential acquisition of transmission and emission images can be used to anatomically standardize the emission image using the same linear parameters as those used for the transmission image. The latter is first reoriented to a template in Talairach space, and the same transformation is then given to the emission images. On the Talairach templates, a predefined volume-of-interest (VOI) set can be constructed (originally performed on perfusion SPECT images), which allows a user-independent sampling of the whole-brain volume of different patients without previously available structural information.
A diagram of the VOI map (22,23) is shown in Figure 1 and a description of VOIs is provided in Table 1. Radioactivity estimates in the cortex were assumed to represent total ligand binding (specific plus nonspecific binding plus free ligand) (18). Because very few 5-HT2a receptors are in the cerebellum compared with the cortex (24), the cerebellar region can be considered to represent nonspecific activity. Calculation of relative indices of specific BI was performed by VOI normalization to the activity per volume element in the cerebellum. Under these pseudoequilibrium circumstances, this BI is directly related to the in vivo receptor density (Bmax) and affinity (KD). BI was defined as:
 which was operationally estimated as:
which was operationally estimated as:
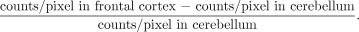
Transaxial, coronal, and sagittal representations of volume-of-interest (VOI) region map oriented in Talairach space as used for study. VOIs represent specific Brodmann’s areas and are shown in different colors. Because of low degrees of freedom, frontal, temporal, parietal, and occipital VOIs were grouped for analysis (Table 1).
Description of Retained Volumes of Interest with Corresponding Brodmann Areas
Statistical Methods
The equality of age and body mass index (BMI) distributions between diagnostic categories was evaluated using the Mann-Whitney test, and the equality of sex distributions was evaluated using the Fischer exact test. As BIs were normally distributed (Kolmogorov-Smirnov testing), t statistics were used to comparemean levels between categories. Correlation analyses were used to examine any relationships among BI, BMI, and disease duration.
RESULTS
Demographic and Physical Variables
The 26 individuals in this study were on average 23.8 y old (SD = 4.1), with ages ranging from 16 to 30 y. Mean ages were not significantly different (Mann-Whitney test = 52.5; P = 0.12) between the 2 study groups, with 22.5 y (SD = 2.5) for patients with AN and 25.6 y (SD = 4.7) for healthy volunteers. A significant difference in sex (Fisher exact test; P = 0.02) existed between the group of healthy volunteers (7 female; 4 male) and patients with AN (15 female).
The BMI in patients with AN was 14.9 (SD = 1.6; range, 11.9–16.9), which was significantly different (Mann-Whitney test = 0; P < 0.0001) from the BMI of the healthy volunteers (mean, 22.3; SD = 1.4; range, 20.5–24.5). The number of years of presence of disease was 4.3 (SD = 4.48; range, 1–14 y). All patients were postmenarcheal and amenorrheic. These variables are summarized in Table 2.
Demographic Variables in Patients with Anorexia Nervosa and Healthy Volunteers
Binding Index
Patients with AN had a significantly reduced 5-HT2a binding potential in the left frontal cortex, in the left and right parietal cortex, and the left and right occipital cortex when compared with healthy volunteers (Table 3). Figure 2 shows an example of a 25-y-old patient compared with the anatomically standardized healthy control population used for this study. Additional adjustment for age did not alter either the magnitude or the statistical significance of the observed difference between the groups.
Example of 25-y-old patient with AN compared with anatomically standardized control population for this study.
Cortical Binding Index in Patients with Anorexia Nervosa and Healthy Volunteers
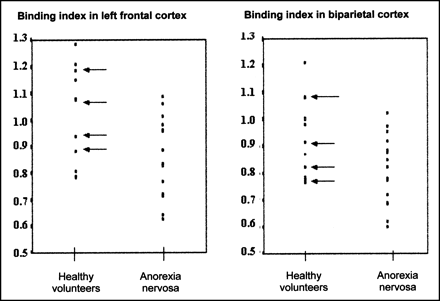
Individual values of left frontal and biparietal BI are plotted in Figure 3. Because of the significant difference in sex distribution in the patients and volunteers, individual values of male volunteers are indicated with arrows. All male volunteers fell in the same range as the female volunteers. Moreover, there is no statistical significance in BI in any of the regions between the male and female healthy volunteers (all P > 0.5).
Plot of individual binding index values in healthy volunteers and patients with AN. Male volunteers are indicated with arrow.
There was a significant difference in the left-to-right ratio in the frontal cortex of patients with eating disorders when compared with healthy volunteers (Table 4).
Left-to-Right Ratios of Binding Index in Patients with Anorexia Nervosa and Healthy Volunteers
Correlation analyses did not reveal significant relationships between regional cortical BIs, BMI, and disease duration.
DISCUSSION
In this in vivo study of cortical 5-HT2a receptors in patients with AN, a significantly reduced 5-HT2a BI in the left frontal, bilateral parietal, and occipital cortices was demonstrated in comparison with that observed in healthy volunteers. A significant difference also was demonstrated in the left-right BI (left < right) in the frontal cortex in patients with AN.
When evaluated in the light of indirect studies assessing serotonergic function and 5-HT2a binding status in low-weight AN patients, the reduction in regional cortical BI is in keeping with the findings of neuroendocrine challenge tests and blood platelet studies that demonstrated a possible 5-HT2 involvement in the pathogenesis of AN (11–13).
The reduction in BI was not present in all cortical regions but was restricted to the left frontal, bilateral parietal, and occipital cortices. This may be in accordance with functional imaging studies that have assessed cerebral blood flow or metabolism in patients with AN and have shown a regional hypometabolism in frontal and parietal cortices (5,6). The occipital cortex was not evaluated in these reports. Neuropsychologic studies have identified a disturbed body image perception in anorectic patients (4), a cognitive function that is attributed to the parietal cortex. Others also have reported deficits in attention (3) and problem-solving abilities (2,3), which are mediated by the frontal cortex, and deficits in digit symbol test (25), visual-spatial construction (2,26), and mental arithmetic (27), which are related to parietal functions.
However, it remains unclear whether this reduction in BI is a cause or a consequence of AN and whether this reduction is trait or state dependent. Many of the biologic findings in low-weight AN patients normalized or were even inversely disturbed after long-term weight restoration (8,10,28) and were state dependent and possibly caused by low weight or neuroendocrine status.
A reduction in 5-HT2a BI can be caused by reduced estrogen concentrations. It has been demonstrated that estrogen increases 5-HT2a receptor expression (29). We did not evaluate estrogen concentrations in the patients in our study. However, because all the women were in a postmenarcheal amenorrheic state, it is probable that estrogen concentration was low and, directly or indirectly, could be the driving force behind the reduction in 5-HT2a expression.
Another important confounding factor is reduced food intake, especially reduced intake of protein, because the essential amino acid tryptophan is the precursor of serotonin. Alternatively, a third possible confounding factor is that a reduced BI can occur as a compensatory response to chronic overrelease of 5-HT. Physical hyperactivity, which is common among AN patients, may cause lipolysis of intermuscular lipids, resulting in the release of free fatty acids. These fatty acids displace tryptophan from albumin, leading to an increase in free tryptophan and in 5-HT turnover in the brain (30). All the aforementioned variables—estrogen, protein reduction, and physical hyperactivity—could be responsible for the reduction in 5-HT2a BI in our study. However, one would then expect a global reduction in 5-HT2a BI, which is contrary to the regional reduction with a clear sparing of the left and right temporal cortex as found in our study.
Our findings also can be confounded by mood status, because depression is a common comorbid disorder among AN patients. This possibility cannot be ruled out; however, no functional imaging study of the 5-HT2a receptor in depressed (31,32) or attempted-suicide patients (20) showed a reduction in parietal BI, a finding that was significantly present in our study. Nor was a frontal asymmetry present in any of these studies of depressed individuals.
As a potential methodologic limitation of our study, the difference in sex distribution may be a possible confounding factor. However, it is unlikely, because sex differences in BI were not reported with this tracer (33), which agrees with our own (unpublished) data, and because male BI values in this study were in the same range as those of healthy female volunteers (Fig. 2).
Because of a lack of structural matching, another potential confounding factor in this study may be the result of partial volume averaging. It is known that substantial (and partially reversible) brain volume loss may accompany low-weight status in patients with AN. Therefore, neocortical activity per unit brain volume may have been underestimated for the patient group. It would be unlikely, however, that such a process is location specific and could account for the regional differences in the frontal and parietal neocortices only.
In this subgroup, no dynamic SPECT acquisitions were made. Hence, the assumption that the imaging outcome measure (the BI) is comparable among patient and control subjects cannot not be made. The pseudoequilibrium conditions for the tracer under consideration were defined with regard to the rate of washout from receptor-rich regions (18). Our own measurements in healthy volunteers (20) have shown that the relative activity in neocortical specific regions with regard to aspecific binding in the cerebellum gradually decreases after 90 min after injection and thereafter can be approximated by a linear decrease during the first hours, with a stable specific-to-aspecific activity ratio. The time of measurement after injection (starting 110–140 min after injection) lies well after the onset of probable pseudoequilibrium. Therefore, although not directly proven in this population, it can be expected that the neocortical equilibrium of the tracer in AN patients would be found approximately at the same time, even with 20%–30% slower kinetics (i.e., reaching of the plateau phase). Moreover, because several neocortical regions (predominantly temporal areas) are normal in patients with AD when compared with those of healthy volunteers, it is unlikely that a very altered kinetic distribution is present within the affected areas that would invalidate the assumption of pseudoequilibrium at the time the measurements were performed after injection.
In addition, without full kinetic modeling, the contribution of altered perfusion and tracer delivery from altered density of binding sites cannot be distinguished. Hence, the possibility cannot be excluded that some portion of the demonstrated changes may involve altered influx to the brain (K1), particularly in light of previous studies in anorectic patients with perfusion deficits in the frontal and parietal neocortices (6,34). Therefore, this study assumes implicitly that the tracer kinetics are in the pseudoequilibrium state after an hour and are not altered by regional changes in K1 but by specific binding properties. Investigations in a small subset of patients with complete kinetic modeling should be conducted to validate this assumption. It should also be taken into account that, in addition to serotonin, other neurotransmitter systems or hormones have altered or disturbed metabolisms in patients with AN. At least theoretically, this is possible with neurotransmitter systems (e.g., norepinephrine and dopamine effects on the serotonergic system) or neurohormonal systems (e.g., cortisol and cortisol-releasing factor effects on the serotonergic system). Because the estimation of the 123I-5I-R91150 BI is based on the assumption of pseudoequilibrium, the effects of these intrinsic and neuronal interactions could alter tracer kinetics.
Another limitation lies in the fact that the specific-to-nonspecific activity ratio is around 2.0 (BI = 1.0) in the age groups studied. The spread in healthy volunteers, which is the combination of intrinsic interindividual physiologic differences as well as uncertainties in tracer delivery (kinetics, distribution, Poisson noise, etc.), is fairly high (18,33), whereas the uncertainty of the BI of 1.0 is about 0.2–0.3 (or 20%–30%). This uncertainty, however, is incorporated in the study, because reference to a group of healthy volunteers was included.
CONCLUSION
A specific regional reduction in BI of the 5-HT2a receptors is demonstrated in low-weight AN patients when compared with healthy volunteers. State-dependent variables of AN may be causal factors in the BI reduction. To address these variables, future work should aim to replicate the findings of this study in the same diagnostic group and in other eating disorder subgroups. A follow-up study of AN patients with a reevaluation of the 5-HT2a BI at long-term weight restoration could help in determining whether this status is state dependent or a trait variable.
Acknowledgments
The authors gratefully acknowledge logistic support from Nuclear Diagnostics Ltd.
Footnotes
Received Feb. 11, 2002; revision accepted Jul. 25, 2002.
For correspondence or reprints contact: Dr. Kurt Audenaert, Department of Psychiatry and Medical Psychology, 1K13 Ghent University Hospital, De Pintelaan 185, Ghent, Belgium.
E-mail: kurt.audenaert{at}rug.ac.be