Abstract
Our aim was to show the ability of a recently developed β+-range–sensitive intracerebral probe (SIC) to measure, in vivo, the binding of radioligands in small animals. Methods: The potential of the device for pharmacokinetic studies was evaluated by measurement of the dynamic striatal binding of 11C-raclopride, a well-documented D2 dopaminergic receptor ligand, in rat brain after intravenous injection of the labeled compound. The effects of preinjection of the unlabeled ligand (raclopride, 2 mg/kg intravenously) and of increasing the synaptic dopamine level (amphetamine treatment, 1 mg/kg intravenously) or of depleting synaptic dopamine (reserpine pretreatment, 5 mg/kg intraperitoneally) on in vivo 11C-raclopride binding were monitored by SIC. Results: The radioactivity curves measured as a function of time were reproducible and consistent with previous studies using PET imaging (ratio of striatum to cerebellum, 2.6 ± 0.3 after 20 min). Further studies showed significant displacement of 11C-raclopride by its stable analog. Finally, the device proved its capacity to accurately detect changes in 11C-raclopride binding after a sudden (amphetamine) or a gradual (reserpine) modulation of endogenous dopamine levels. Conclusion: These results show that the new device can monitor binding of PET ligands in anesthetized rodents in vivo, with high temporal resolution.
The development of new cerebral radiopharmaceuticals labeled with β+-emitters, as well as the functional characterization of currently available neuroligands, requires validation experiments on laboratory animals, often combining postmortem tissue sampling with ex vivo autoradiography (1,2). These ex vivo studies are time consuming and require the killing of several animals to properly measure tracer kinetics. This difficulty has led, in the past few years, to the development of high-spatial-resolution tomographic systems for in vivo imaging of rodents (1,3,4). However, notwithstanding promising results (5–7), these tools suffer from several major drawbacks. They are expensive, and their temporal resolution is limited to a few minutes between each data point because of the high counting statistics required for image reconstruction. A complementary and alternative approach has therefore been proposed, consisting of a sensitive intracerebral probe (SIC, an acronym for the French term “sonde intracérébrale”). It is a β+-range–sensitive microprobe directly implanted in the brain that enables local counting of radioactivity in a restricted and stereotactically well-defined brain region. This tool is not an imaging device but a counter of radioactivity. The physical characterization and initial in vivo evaluation of SIC in the anesthetized rat have recently been described (8).
The aim of this study was to further show the potential of this new intracerebral probe for in vivo neuropharmacologic experiments in rats. A well-characterized PET ligand, 11C-raclopride, was chosen as the labeled form of a specific reversible antagonist of the dopaminergic D2 receptor. The striatum is the brain structure richest in this type of receptor. In contrast, the cerebellum is devoid of D2 receptors. Accordingly, the experiments were conducted to determine the time–concentration curves for 11C-raclopride in the striatum and cerebellum and the effect of presaturation with nonlabeled raclopride on specific binding, the reliability of the device as evaluated both by interindividual comparisons and by multiple injections on the same animal, and the feasibility of using SIC to study the effects of altered extracellular dopamine (the endogenous ligand) on raclopride binding. With this aim, we studied the effects of increasing the synaptic dopamine level (amphetamine treatment) or of depleting synaptic dopamine (reserpine pretreatment) on in vivo 11C-raclopride binding monitored by SIC.
Competition between endogenous dopamine and exogenous radiolabeled ligands for binding to dopamine D2 receptors is well documented in rodents. An amphetamine injection leads to massive release of dopamine and blockade of reuptake, thereby increasing the extracellular dopamine concentration. This increase is known to reduce in vivo binding of the D2 antagonist 11C-raclopride in rodents (9–11), in nonhuman primates (12–15), and in humans (16–18). The opposite effect (i.e., increased 11C-tracer accumulation) is obtained by reserpine, a drug that decreases the endogenous concentration of dopamine (15,19,20).
MATERIALS AND METHODS
Design of SIC
The SIC apparatus was conceived and manufactured at the Institut de Physique Nucléaire (Orsay, France). The detection principle of the probe has already been described (8). The detector takes advantage of the limited range of β-particles within biologic tissues to determine a limited detectable thickness surrounding the probe (21). Monte Carlo simulations, confirmed by calibrations on phantoms (8), showed that for 11C-labeled radiotracers, 90% of the counting rate measured by the probe corresponds to β+-particles emitted at a distance of 2.05 mm from the tip of the probe. The sensitive end of the probe consists of a 1-mm-long and 1-mm-diameter plastic scintillating fiber (BCF-12; Bicron, Newbury, OH). This detection tip is fused with a 1-mm-diameter clear optic fiber (BCF-98; Bicron) whose length is adjustable to the depth of the brain structure targeted (e.g., 7 mm in the case of an implantation in rat striatum). This feature enables one to detect the light exiting the brain. The light is then transmitted through an optic fiber to a photomultiplier (R7400P; Hamamatsu, Hamamatsu City, Japan). The electronic pulses are analyzed by a counting electronic device, and the measured counting rate is displayed online with LabVIEW software (National Instruments, Austin, TX). The response of the detector was shown to be linear over the whole scale of radioactivity used in this study. The probe sensitivity was experimentally determined to be 0.68 cps/kBq/mL. This sensitivity leads, with a 37-MBq (1 mCi) injected dose, to counting rates on the order of a few hundred counts per second (depending on the specificity of the tracer to its biologic target), thus allowing us to set the integration time to 1s.
Synthesis of 11C-Raclopride
11C was produced by the nuclear reaction 14N(p,α) using a cyclotron (Cyclone 18/9; Ion Beam Applications, Louvain-la-Neuve, Belgium) at the PET center (Lyon, France) and obtained as 11C-CO2. 11C-raclopride was synthesized by O-methylation of its precursor, (S)-3,5-dichloro-N-((1-ethyl-2-pyrrolidinyl)methyl)-6-methoxysalicylamide (provided by Astra, Södertälje, Sweden), with 11C-methyl iodide. Quality control tests were completed before injection. The radiochemical and chemical purities used were greater than 98%, and the specific radioactivity at the time of injection ranged from 66.6 to 129.5 GBq (1.8–3.5 Ci)/μmol.
Surgery and SIC Implantation
Nineteen male Sprague–Dawley rats (Iffa Credo, L’Arbresle, France) weighing 300–400 g were housed under standard conditions of temperature and humidity and, from 8 am to 8 pm, under an artificial light. All experimental procedures were in accordance with the guidelines of European Union Council directory 86/09/EEC. The rats were anesthetized by a single intraperitoneal injection of urethane (Sigma-Aldrich, Saint Quentin, France) at a dose of 1.7 g/kg of body weight and remained anesthetized throughout all procedures. After catheterization of a tail vein, the rats were positioned on a stereotactic apparatus (Unimecanique, Epinay, France), the skull was exposed, and the bregma point was found. One SIC was implanted in the striatum (Fig. 1), and a second SIC was implanted in the cerebellum. According to the atlas of Paxinos and Watson (22), the coordinates of implantation (in millimeters) in the striatum were 1.0 for anteroposterior (A/P), 2.5 for lateromedial (L/M), and −6.0 for ventrodorsal (V/D) and in the cerebellum were −12.0 for A/P, 3.0 for L/M, and −3.0 for V/D. The A/P reference was the bregma point, and the V/D reference was the dura. Body temperature was maintained at 37°C ± 1°C throughout the test, using a thermostatically controlled heating blanket (CMA 150; CMA Microdialysis, Solna, Sweden).
Localization of SIC (arrow) in rat striatum on 20-μm cryostat-cut section of rat brain colored with cresyl blue (A) and on schematic representation of brain structures in sagittal plane corresponding to probe implantation (B). Cc = corpus callosum; CP = caudate putamen complex; Cx = cortex.
SIC Scanning Procedure
SIC data were acquired 2 h after implantation of the probes (to allow a stabilization period). For each acquisition, 74 MBq (2 mCi) 11C-raclopride (in 0.5 mL saline) were injected in the tail vein over 45 s, and the time course of radioactivity was studied for 100 min using a 10-s time integration acquisition. The following 3 protocols were applied.
Protocol 1 (Raclopride Saturation).
Three hours after the initial acquisition, 5 rats received an injection of the corresponding stable compound (2 mg/kg intravenously) 10 min before a second 74-MBq injection of 11C-raclopride. Two animals also received the 2 successive injections of 11C-raclopride without first receiving the stable compound.
Protocol 2 (Amphetamine Displacement).
Thirty minutes after 11C-raclopride injection, amphetamine was injected intravenously over 30 s (1 mg/kg in 300 μL saline) in the vein catheter used for radioligand administration.
Protocol 3 (Reserpine Displacement).
Reserpine (5 mg/kg dissolved in a saline solution containing 3% glacial acetic acid) was administered intraperitoneally 24 h before injection of the radiotracer.
Probe Placement Controls
After the acquisition, the anesthetized rats were killed and the brain was quickly removed and frozen at −80°C. Coronal tissue sections 20 μm thick throughout the striatum and cerebellum were cut in a cryostat at −20°C and were thaw mounted onto glass slides. The sections were colored by cresyl blue, and the placement of the probes was atlas matched.
Data Analysis
SIC data (expressed as mean number of disintegrations per 10 s) were averaged every minute. These data were corrected for radioactive decay and were normalized with both the total activity injected and the specific radioactivity of the 11C-raclopride. Statistical analysis was conducted by comparing the means obtained from the control and treated animals for each time value (every minute) and using the unpaired Student t test.
RESULTS
A typical histologic control of the probe location is presented in Figure 1. All the time–radioactivity curves presented are corrected for isotope decay.
Protocol 1
The averaged measures of radioactivity obtained as a function of time after the first injection of 11C-raclopride to 5 untreated animals are presented in Figure 2. The graph shows that the radioactivity was significantly lower in the cerebellum than in the striatum (30 ± 7 disintegrations per second [dps] vs. 88 ± 15 dps, 30 min after injection; P < 0.05). The striatum-to-cerebellum ratio was 2.6 ± 0.3. Both curves decreased 20 min after the injection, and washout in both the cerebellum and the striatum proceeded with an almost identical slope.
Striatal and cerebellar time–activity curves after bolus intravenous administration of 74 MBq (2 mCi) 11C-raclopride. Each point is mean (+SEM) of radioactivity (in disintegrations per second) found in 5 animals. Arrow indicates time of radioligand injection. *P < 0.05 using unpaired Student t test. dps = disintegrations per second.
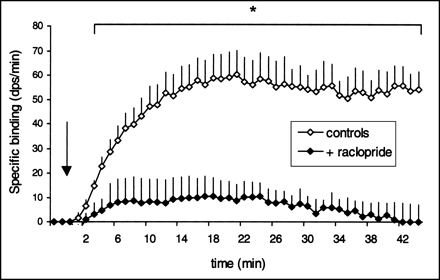
The averaged measures of the striatal specific binding of 11C-raclopride calculated from SIC data after intravenous injection, with or without an unlabeled raclopride pretreatment, are presented in Figure 3. Specific binding in the striatum was obtained by subtracting the activity found in the cerebellum (nonspecific binding) from that found in the striatum (specific + nonspecific binding). The top curve corresponds to a single injection of 11C-raclopride (control) in 5 rats. The bottom curve corresponds to the second 11C-raclopride injection, which was preceded (10 min) by an injection of unlabeled raclopride (2 mg/kg intravenously). In the latter case, no specific binding was detectable in the striatum. When the injection of unlabeled ligand was omitted before the second 11C-raclopride injection, both curves gave similar results (n = 2, data not shown).
Specific binding of 11C-raclopride in striatum after injection of stable raclopride (+ raclopride) compared with specific binding of 11C-raclopride in absence of saturating dose of stable raclopride (controls). Intravenous injection of 2 mg/kg stable raclopride was followed (10 min) by bolus injection of radioligand (arrow). Specific binding was obtained by subtracting radioactivity found in cerebellum from radioactivity found in striatum. Mean values (+SEM) for each group were significantly different when controls (n = 5) and treated animals (n = 5) were compared with unpaired Student t test. *P < 0.05.
Protocol 2
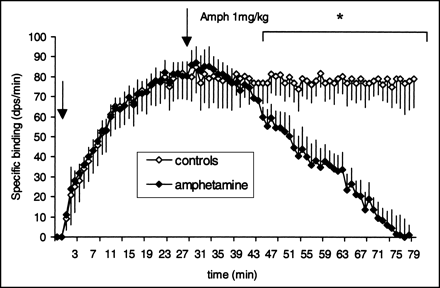
The mean value of binding in the cerebellum did not significantly differ between the rats that received amphetamine and the control rats. On the contrary, amphetamine administration resulted in a rapid and significant decrease in striatal specific binding. Figure 4 shows striatal specific binding of 11C-raclopride before and after an intravenous injection of amphetamine (1 mg/kg) in 5 rats. The binding decreased progressively after amphetamine administration and differed significantly from that in controls 20 min after the treatment (P < 0.05). Fifty minutes after the amphetamine injection, no specific binding could be detected in the striatum.
Alteration of striatal specific binding (+SEM) measured as described in Figure 3 after intravenous injection of 1 mg/kg amphetamine (second arrow). Mean values for each group were compared for each time. Significant differences were found between controls (n = 5) and amphetamine-treated animals (n = 5) with unpaired Student t test. Amph = amphetamine; *P < 0.05.
Protocol 3
Mean specific binding in 5 rats pretreated with reserpine (5 mg/kg intraperitoneally) 24 h before the experiment are presented in Figure 5. Mean binding in the cerebellum did not significantly differ between the rats that received reserpine and the control rats. Reserpine administration resulted in a significant increase in striatal specific binding in comparison with that in control rats (P < 0.05). The rats that received reserpine showed a significant increase in the striatum-to-cerebellum ratio (3.8 ± 0.3) 30 min after 11C-raclopride injection (P < 0.05).
Striatal specific binding of 11C-raclopride (+SEM, n = 5) 24 h after intraperitoneal injection of reserpine (5 mg/kg; arrow). Controls (n = 5) were compared using unpaired Student t test. *P < 0.05.
DISCUSSION
The aim of this study was to validate the efficacy of SIC in characterizing the in vivo binding kinetics of radiopharmaceuticals in small laboratory animals. The results show the validity and feasibility of using SIC for 11C-raclopride studies in rats. 11C-raclopride was chosen because of its wide use as a D2 receptor ligand in PET studies and because its radiopharmacologic properties are well documented (23). In animal experiments, raclopride has been shown to be a potent and selective antagonist of dopamine D2 receptors (24,25). Dopamine receptors are nonuniformly localized in the brain. The reference region usually chosen for measurement of the dopamine system is the cerebellum, because of its low density of dopamine receptors (26,27). In the current experiments, we assumed that regional radioligand delivery and nonspecific binding did not differ. Thus, specific binding to the D2 receptors was estimated to be the difference in concentration of radioligand between the reference region (cerebellum, poor in D2 receptors, nonspecific binding) and the region of interest (striatum, rich in D2 receptors, specific + nonspecific binding).
11C-raclopride radioactivity curves are well documented for PET scans (28). After intravenous administration, 11C-raclopride accumulates preferentially in the striatum, with a ratio of approximately 3 with the cerebellum (29). Moreover, repeated measures of 11C-raclopride binding in human or animal brain are known to be highly reproducible (10,11,14,15,17).
In our study, the striatum-to-cerebellum ratio increased linearly with time, reaching a value of 2.6 ± 0.3 after 20 min and being reproducible between rats, in good accordance with previous PET studies (9,10). No initial peak of radioactivity in the striatum or in the cerebellum was detected with SIC, in contrast with the peak observed in PET scans. The initial peak of radioactivity is generally considered as the vascular bolus. Its absence in SIC data suggests that SIC is less sensitive to the circulating radioactivity than are PET cameras.
To ensure that, in the raclopride displacement protocols, the long-term anesthesia did not influence 11C-raclopride binding, we gave a double injection to a single rat at 2 and 5 h after anesthesia, without displacement. The binding characteristics of raclopride were the same, revealing that, in our protocol, urethane had no significant effect on ligand binding, in accordance with previous studies showing that urethane does not have a known effect on the dopaminergic system or on cerebral blood flow (30). In rats that first received stable raclopride and, thus, had sites partially to fully occupied at the time of radioligand injection (24), mean striatal binding was reduced to approximately the value obtained in the cerebellum. This finding confirms that the radioactivity measured in the striatum and exceeding the value found in the cerebellum is well due to specific binding and sustains the proposal that nonspecific binding in the striatum is similar to that in the cerebellum.
It was important to ensure that SIC is sensitive to the endogenous competition between dopamine and 11C-raclopride binding. The sensitivity of 11C-raclopride binding to competition from endogenous dopamine is partly granted by the low affinity of raclopride for dopamine D2 receptors (24,31). PET studies show that administration of pharmacologic agents known to alter extracellular dopamine can affect binding of 11C-raclopride. In particular, substances known to increase dopamine, such as amphetamine, have been shown to lead to a decrease in D2 ligand binding (11,32,33). Analysis of PET studies in primates and microdialysis studies in rats has shown that an induced change in D2 ligand binding corresponds to an opposing change in dopamine level (34). The alteration of 11C-raclopride binding in vivo as measured by PET might not, however, simply be modulated by the apparent extracellular concentration of dopamine (14). In agreement with these previous PET studies using 11C-raclopride, our SIC data confirm that amphetamine administration may lead to a significant decrease in striatal specific raclopride binding (−100% on average, 50 min after amphetamine administration). It would be interesting to correlate (in the same animal) amphetamine-induced dopamine changes (by microdialysis) and 11C-raclopride binding (with SIC) to show the relationship between extracellular dopamine concentration and 11C-raclopride specific binding. Such a study is in progress.
On the other hand, in agreement with previous studies using 3H-raclopride in rodents (9,19), depletion of dopamine stores by reserpine leads to an increase in the specific binding of raclopride to D2 dopamine receptors. Reserpine is known to deplete dopamine vesicles after blockade of the vesicular monoamine transporters and to durably reduce extracellular dopamine (35,36). Because the depleting effect of reserpine is too gradual to significantly modify 11C-raclopride binding during the first 1.5-h acquisition, we performed the injection 24 h before the 11C-raclopride experiment, according to (9) and (37). Furthermore, using PET of the monkey striatum, Ginovart measured a significantly increased 11C-raclopride binding 48 h after injection of reserpine (15). In accordance with these previous studies, the current results show that SIC was able to detect, reliably, the enhanced 11C-raclopride specific binding in striatum. As a consequence, this study shows that SIC is sufficiently sensitive to changes in binding that may be detected after a sudden (amphetamine) or gradual (reserpine) modulation of endogenous dopamine levels.
Although our SIC data are comparable with PET-scan curves, some aspects of the SIC conception distinguish it from a classic PET camera. By its principle, SIC does not deliver images but defines only a detection volume drawn around the probe, which is correlated to the energy spectrum of the chosen isotope. Because the stereotactic implantation and the range of β-particles in tissue are well controlled, SIC is well suited to the size of rat brain loci. Moreover, stereotactic implantation will improve with the use of 500-μm and 200-μm probes, which are under study. Another characteristic of SIC is that it allows visualization of time–radioactivity curves online (minimum time integration, 1 s), allowing modification of the protocol during the experiments. This real-time sampling is compatible with the metabolism period of a tracer and with online analysis of neuropharmacologic processes or tracer input functions.
The use of SIC, in its current conception, requires animal anesthesia, the effects of which must be estimated and considered in the interpretation of any pharmacokinetic data. However, our results encourage investigators to further develop the current device for use on freely moving rats by cementing probes on the skull of the animal and adapting a rotating set on top of the cage. Thus designed, the SIC device, described here for the first time, may easily be combined with PET or microdialysis, giving an association of major interest in cerebral ligand research.
CONCLUSION
In this study, we evaluated the radiopharmacologic performance of the first complete prototype of a new detector dedicated to measurement of radiotracer kinetics in an area of small-animal brain. The detector was based on a small scintillating probe coupled to an ultra compact and low-noise photomultiplier tube. The SIC results show that its sensitivity and selectivity allow its use in studies of the kinetics of established or potential PET ligands in small animals. Furthermore, the direct data visualization possible with SIC constitutes a simplification over PET protocols. In the current study, use of the device was limited to striatal dopaminergic function. Application of the methodology to either more uniformly or less densely localized neurotransmitter systems remains to be validated.
Footnotes
Received Jan. 29, 2001; revision accepted Jul. 10, 2001.
For correspondence or reprints contact: Luc Zimmer, PharmD, PhD, Centre d’Exploration et de Recherche Médicales par Émission de Positons, Biomedical Cyclotron, 59 Boulevard Pinel, F-69003 Lyon, France.
E-mail: zimmer{at}cermep.fr