Abstract
Parkinson’s disease is a progressive neurodegenerative disorder characterized by a selective loss of dopamine in the striatum. Problems remain in the accurate diagnosis of Parkinson’s disease. A 99mTc-labeled tropane derivative that binds to dopamine transporter with high selectivity is [2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3,2,1]oct-2-yl]methyl](2-mercaptoethyl)amino]ethyl]amino]ethanethiolato(3-)-N2,N2′,S2,S2′]oxo-[1R-(exo-exo)] (TRODAT-1). The purpose of this study was to investigate the potential usefulness of 99mTc-TRODAT-1 imaging in the evaluation of patients with early-stage Parkinson’s disease. Methods: Thirty-four patients with early-stage idiopathic Parkinson’s disease were recruited. For all patients, the Parkinson’s disease was stage 2 or less as assessed by the Hoehn and Yahr scale. Seventeen age-matched healthy volunteers (8 men, 9 women) served as controls. 99mTc-TRODAT-1 was prepared from a lyophilized kit. Brain SPECT imaging was performed between 165 and 195 min after injection, using a double-head camera equipped with fanbeam collimators. Specific uptake in the striatum and its subregions, including the putamen and caudate nucleus, was calculated and compared with that of the other sides and of healthy volunteers. Results: A continuous reduction in specific striatal uptake of 99mTc-TRODAT-1 with increasing disease severity was found in Parkinson’s disease patients (control vs. stage I vs. stage II, 1.98 vs. 1.62 vs. 1.22, respectively, P < 0.01). The changes were magnified by measurement of specific putaminal uptake (control vs. stage I vs. stage II, 1.81 vs. 1.27 vs. 0.94, respectively, P < 0.01). The mean values of specific putaminal uptake contralateral to the more affected limbs were significantly decreased compared with the ipsilateral sides in both stage I and stage II groups (1.02 vs. 1.49 for stage I and 0.73 vs. 1.14 for stage II, P < 0.01). Moreover, a significant loss of putaminal uptake ipsilateral to the symptoms was found in the stage I group compared with the healthy volunteers (1.49 vs. 1.81, P < 0.01). The difference became greater when the posterior putaminal uptakes were compared. No remarkable adverse reactions were found in either healthy volunteers or Parkinson’s disease patients during or after imaging. Conclusion: For clinical practice, 99mTc-TRODAT-1 may serve as a useful imaging agent for the early detection of Parkinson’s disease.
Parkinson’s disease is primarily caused by a progressive decrease of dopaminergic neurons in the nigrostriatal pathway and is characterized by an insidious onset of motor symptoms such as rigidity, tremor, and bradykinesia (1). An accurate diagnosis of Parkinson’s disease is important not only for counseling and management of patients but also for conducting pharmacologic and epidemiologic studies. At present, the diagnosis of Parkinson’s disease still depends on clinical criteria (1). Recent studies showed that an unexpectedly high rate of misdiagnosis occurred if the diagnosis was based on only the clinical diagnostic criteria (2). In addition, motor disturbances began only after a loss of approximately 70%–80% of striatal dopamine, resulting in a long “latent” stage preceding the development of clinical manifestations (3). These facts imply that the accuracy of diagnosing Parkinson’s disease needs improvement.
PET and SPECT have enabled noninvasive, in vivo visualization of the progression of striatal neuronal function in Parkinson’s disease patients (4). The dopamine transporter (DAT) is located in the presynaptic membrane on the terminal of the dopaminergic projection (5) and is thought to be a marker of dopamine terminal innervation (6). Degeneration of the projection from the substantia nigra to the striatum resulted in loss of DATs (5,7). Several 123I-labeled DAT SPECT imaging agents based on cocaine or the closely related tropane derivatives have been reported (4,8,9). Using 123I-2β-carbomethoxy-3β-(4-iodophenyl)tropane (β-CIT) SPECT, Marek et al. (8) and Staffen et al. (10) identified patients with Parkinson’s disease at the onset of motor symptoms and suggested that this technique may be useful for identifying—before the onset of motor symptoms—individuals in whom dopaminergic pathology is developing. However, because of its limited availability and relatively high cost, 123I-labeled DAT ligands will play only a small role for studying DAT in the clinic (11).
99mTc has an optimal energy for imaging, is much cheaper than other available radionuclides, and is available in every clinical nuclear medicine facility. Therefore, 99mTc-labeled ligands are more suitable for routine imaging. Several 99mTc-labeled tropane analogs have been synthesized (11–14). Among these, a 99mTc-labeled tropane derivative, [2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3,2,1]-oct-2-yl]methyl](2-mercaptoethyl)amino]ethyl]amino]ethanethiolato(3-)-N2,N2′,S2,S2′]oxo-[1R-(exo-exo)] (TRODAT-1), was found to be a potential agent for imaging DATs (14–18). Its binding characteristics and pharmacologic specificity have also been realized in rats and in primate brain in vitro and in vivo (11,13,19). Because DATs are located only on dopaminergic nerve terminals (20), postmortem studies have shown a close relationship between DAT concentrations and striatal dopamine levels (21,22) and the presence of Parkinson’s disease (15,23). Thus, DAT imaging may provide a measure of dopamine terminal innervation of the striatum. The current study was performed to test the feasibility of using 99mTc-TRODAT-1 and conventional nuclear medicine facilities as tools for evaluating patients with early-stage Parkinson’s disease.
MATERIALS AND METHODS
Subjects
Thirty-four patients with early-stage Parkinson’s disease were studied. Parkinson’s disease was diagnosed according to generally accepted criteria (24). The severity of Parkinson’s disease was assessed using the Hoehn and Yahr scale (HYS) (25). In addition, the patients we selected were >40 y old and response was to a single-dose levodopa challenge. Fifteen patients were in HYS stage I (7 men, 8 women; age range, 47–78 y; mean age ± SD, 62 ± 9 y), and 19 were in HYS stage II (10 men, 9 women; age range, 54–79 y; mean age, 67 ± 8 y). Specific uptake in the striatum, putamen, and caudate nucleus was measured bilaterally. The mean activity of the more affected side (contralateral to the more affected limbs) was compared with that of the other side and of healthy volunteers. Seven patients were drug naive. The other patients had taken antiparkinsonian medication (l-dopa) for various periods but had discontinued it at least 24 h before and until completion of the studies.
Seventeen age-matched healthy volunteers (8 men, 9 women; age range, 45–76 y; mean age, 63 ± 9 y) served as controls. On the basis of a screening interview, none of the healthy volunteers had a history of neuropsychiatric disorders or a family history of movement disorders. For at least 3 mo, none had taken medication known to affect brain function.
All patients and healthy volunteers gave written informed consent. The study was approved by the ethical committees of our hospital and the Department of Health of the Republic of China.
Radiopharmaceuticals
99mTc-TRODAT-1 was prepared as has been reported (11), with minor modifications. Briefly, it was prepared from a lyophilized kit by adding 1,110 MBq freshly eluted 99mTc-pertechnetate to 5 mL saline preparation (17). The 99mTc-TRODAT-1 was obtained in a neutral solution (pH, 7.0–7.5) with >90% radiochemical purity over 6 h as determined by high-performance liquid chromatography. The shelf life of the lyophilized kit was >2 mo when it was stored at room temperature.
Imaging and Data Acquisition
All subjects consumed a low-protein diet for 24 h before the 99mTc-TRODAT-1 examinations. The subjects were placed supine, with their head fixed in a holder. After a single bolus injection of 740 MBq 99mTc-TRODAT-1, 15 dynamic images of the brain were acquired during 30 min (2 min per frame). The brain SPECT images were then acquired between 165 and 195 min after injection using a double-head camera equipped with fanbeam collimators (Helix SPX; Elscint, Haifa, Israel). Data were acquired in a 128 × 128 matrix with a 1.4 zoom through 360° (180° for each head) rotation at 3 intervals, for 30 s per angle step. Images were reconstructed using backprojection with a Metz filter. Attenuation correction was performed by the first-order method of Chang. The SPECT images were analyzed along the level of the canthomeatal line. Regions of interest were marked for one side of the striatum in reference to the corresponding MR image and were fitted to the other side. Regions of interest were drawn over the whole striatum, putamen, and caudate nucleus of each hemisphere on composite images of the 3 slices with the highest basal ganglia activity. The occipital cortices (OC) were also drawn in the same way and served as background areas. The specific uptake was calculated by subtracting the mean counts per pixel in the OC from the mean counts per pixel in the whole striatum, putamen, or caudate nucleus and dividing the result by the mean counts per pixel in the background, that is, (striatum − OC)/OC, (putamen − OC)/OC, or (caudate nucleus − OC)/OC. The posterior putaminal uptake in healthy volunteers and on the ipsilateral side in patients with stage I Parkinson’s disease was also analyzed (26).
Statistics
Multiple ANOVA for multigroup comparisons was performed using SPSS 9.0 software (SPSS Inc., Chicago, IL) for Windows (Microsoft, Redmond, WA). If the result was significant, the Dunnett multicomparison test was used to compare the normal mean value with the values of the other groups. The Student t test for paired samples was performed for within-group comparison of outcome measures between ipsilateral and contralateral sides and between those of the healthy volunteers. Significance was defined as P < 0.05. Results are reported as the mean ± SD.
RESULTS
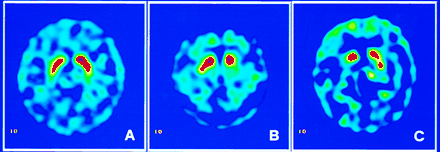
The images were interpreted both visually and by semiquantitative analysis. In the dynamic studies, we found that radioactivity accumulated in the basal ganglia area of each subject. On SPECT images, a better contrast of radioactivity between the striatum and adjacent brain tissue was observed in healthy volunteers than in patients (Fig. 1).
99mTc-TRODAT-1 SPECT in transverse views of healthy volunteer (A) and of patients with HYS stage I (B) and stage II (C) Parkinson’s disease. Asymmetric striatal uptake was observed, with more profound loss contralateral to symptoms or more affected limbs.
Significant differences in specific striatal uptake were found among healthy volunteers, patients with stage I disease, and patients with stage II disease (1.98 ± 0.24, 1.62 ± 0.11, and 1.22 ± 0.12, respectively, P < 0.01), as shown in Table 1 and Figure 2. Greater loss of uptake occurred in the putamen than in the caudate nucleus and contralateral to the more affected limbs, although striatal uptake was bilaterally reduced. The specific putaminal uptake decreased markedly in Parkinson’s disease patients (control vs. stage I vs. stage II, 1.81 ± 0.18 vs. 1.27 ± 0.14 vs. 0.94 ± 0.20, P < 0.01) (Table 1; Fig. 3). The average decrease in putaminal uptake contralateral and ipsilateral to symptoms or more affected limbs was 44% and 18%, respectively, in stage I and 60% and 37%, respectively, in stage II (Table 1; Figs. 4 and 5). Although overlap still existed in 8 cases, a significant loss of putaminal uptake ipsilateral to the symptoms was found in the stage I group compared with the healthy volunteers (1.49 ± 0.22 vs. 1.81 ± 0.18, P < 0.01). The difference became greater when the posterior putaminal uptakes were compared (1.29 ± 0.17 vs. 1.67 ± 0.15), and in only 4 cases was there overlap (Fig. 6).
Specific striatal uptake of 99mTc-TRODAT-1 in healthy volunteers and in patients with HYS stage I and stage II Parkinson’s disease.
Specific putaminal uptake of 99mTc-TRODAT-1 in healthy volunteers and in patients with HYS stage I and stage II Parkinson’s disease.
Specific putaminal uptake of 99mTc-TRODAT-1 in healthy volunteers and in patients with HYS stage I Parkinson’s disease contralateral and ipsilateral to symptoms.
Specific putaminal uptake of 99mTc-TRODAT-1 in healthy volunteers and in patients with HYS stage II Parkinson’s disease contralateral and ipsilateral to symptoms.
Specific posterior putaminal uptake of 99mTc-TRODAT-1 in healthy volunteers and in patients with HYS stage I Parkinson’s disease ipsilateral to symptoms.
Specific Uptake of 99mTc-TRODAT-1 in Striatum and Subregions in Patients with Early Parkinson’s Disease
No significant change in mean uptake by the caudate nucleus—either in its entirety or for each side considered separately—was observed between stage I patients and healthy volunteers, but a significant decrease was seen in stage II patients (2.13 ± 0.31 vs. 1.51 ± 0.17, P < 0.01) (Table 1). No remarkable adverse reactions were found in either healthy volunteers or Parkinson’s disease patients during or after imaging.
DISCUSSION
In patients with early-stage Parkinson’s disease, a significant loss of striatal uptake of 99mTc-TRODAT-1 at 165–195 min after injection was found using conventional double-head nuclear medicine facilities. The profound differences in specific uptake in the striatum and especially in the putamen in early-stage Parkinson’s disease enabled us to evaluate patients by visual inspection of images. The significant reduction of putaminal uptake ipsilateral to the symptoms, especially posteriorly, in stage I patients may provide a clue for following disease progression. Our results support previous observations using either SPECT (8,14,27,28) or PET (21,29) and suggest that 99mTc-TRODAT-1 may be a useful agent for DAT imaging to assess early dopaminergic neuron loss. In addition, 99mTc-TRODAT-1 DAT imaging appears to be safe for humans (30).
The diagnosis of Parkinson’s disease still relies mainly on observation of typical clinical symptoms and signs. Notably, the accuracy of the clinical diagnosis of Parkinson’s disease is approximately only 70%–80% on the basis of clinicopathologic studies (1). It is therefore crucial to find a clinically feasible way to more easily evaluate the etiology and severity of the disease. Prior studies have shown that PET can determine the integrity of the dopaminergic neurons in vivo and thus play an important role in the early diagnosis and differentiation of so-called parkinsonian syndromes (4,31,32). In addition, many promising studies using SPECT imaging with 123I-labeled tracers have been reported. Because 123I is produced in a cyclotron, its high cost and lack of availability in most nuclear medicine departments limit its routine clinical use. Kit-based 99mTc-labeled radiopharmaceuticals combined with conventional diagnostic cameras provide an ideal means for routine use. In this study, we used a double-head camera equipped with a fanbeam collimator, both of which are commonly available worldwide. Such a combination fulfills the requirements for widespread clinical application.
Progress in neuroscience has made evident that nigral dopaminergic projections to the striatum, especially those to the putamen, are targeted in Parkinson’s disease, whereas those to the caudate nucleus are relatively spared (5,7,10). Our results agree with previous SPECT and PET studies that showed a correlation between DAT density and disease severity (8,16,29). On the other hand, pathologic evidence has disclosed a more severe depletion of dopamine in the putamen than in the caudate nucleus in Parkinson’s disease patients (4,5,8,27), in contrast to many Parkinson’s disease–like disorders, which usually show a more uniform and symmetric striatal loss of dopaminergic activity (4,10,33). These histopathologic differences provide an assessable approach for the early diagnosis of Parkinson’s disease and perhaps can be used to discriminate etiologies of parkinsonism by measuring specific putaminal uptake, especially in the posterior portion (8,15,27,34). In this study, we found an obvious loss of putaminal uptake contralateral to the more affected limbs. The specific putaminal uptake contralateral to symptoms in the stage 1 group was also significantly reduced compared with the ipsilateral side and the healthy volunteers. These results agree with recent studies showing that binding of 99mTc-TRODAT-1 to DAT is sensitive to minimal changes in the availability of DAT (15,16,20). The greater decrease of 99mTc-TRODAT-1 uptake in putamen than in caudate nucleus may enhance the sensitivity of 99mTc-TRODAT-1 imaging for early detection of Parkinson’s disease (15,16).
Interestingly, compared with the healthy volunteers, patients in the stage I group also had a significantly decreased uptake in putamen ipsilateral to the symptomatic side. This difference became more pronounced when the comparison focused on the posterior putamen, suggesting that measuring posterior putaminal uptake may lead to a better separation between stage I ipsilateral uptake and uptake in healthy individuals. This finding agrees with results from necropsy studies (5,35). Similar results were observed using 123I-β-CIT (8), 123I-IPT (27), and 99mTc-TRODAT-1 (15), suggesting that DAT imaging may be useful for detecting the loss of DATs before the onset of symptoms. As shown in this study, both total striatal and putaminal uptake reflected disease severity. These results also imply that initial unilateral involvement will eventually become bilateral with disease progression (25). Such an approach, to our knowledge, is not easily obtainable with any other imaging modality. In addition, Ahlskog (36) reported that pronounced depletion of presynaptic dopaminergic nigrostriatal terminals seems to be a necessary prerequisite for the short-duration levodopa response. Therefore, the clinical applications of 99mTc-TRODAT-1 imaging may not be limited to disease diagnosis but may also play a role in the management of Parkinson’s disease patients in the future.
The decline of DAT density was found to be independent of sex (37). However, the effect of age on DAT density in humans is still controversial. Muller et al. (37) found no significant effect of age on relative or absolute striatal uptake in their Parkinson’s disease patients. Others, however, found that DATs decrease with age (16,17,38), whereas Mozley et al. (15,39) observed that the effect does not appear to be linear, with the rate of change slowing over the age of 40 y. Accordingly, we selected both healthy volunteers and patients > 40 y old. Mean age did not significantly differ among the groups. Although we discontinued l-dopa treatment for 24 h before imaging to avoid possible effects on the binding of 99mTc-TRODAT-1 to DAT, a recent observation indicates that the doses normally used for Parkinson’s disease patients are unlikely to alter the results of SPECT imaging (11).
Although SPECT with triple-head systems has been widely accepted for investigating the neuronal metabolism of dopamine in Parkinson’s disease, this and other studies (8,9,37) show that double-head cameras, which are used worldwide, may also provide a clinically convenient and cost-effective way to detect Parkinson’s disease.
We chose the OC as a reference region because of the low density of monoamine transporters there (8,14) and the ability to easily compare data between the striatum and the OC from the same slice. The OC is also more convenient for comparisons with the corresponding MR image (canthomeatal line). However, controversy remains about use of the OC or cerebellum as reference regions, because these small regions sometimes have low counting rates (15). Although different reference regions have been used for DAT studies (14–18,27), measuring the ratio of striatal activity to OC activity is simple and easy to apply in daily clinical studies (17,18,40). We performed a dynamic study more than 30 min after injection to ensure proper dose administration and allow the compound to penetrate the blood–brain barrier and localize in the brain (14). Loss of brain uptake in early imaging usually represented failure of delayed SPECT imaging (14). Although reduced striatal uptake was observed by us and many others, the semiquantitative data in this study varied from those of previous reports. This variance may have been caused by differences in the method of tracer synthesis (15–17), in the brands and models of technical equipment (14–16), in the reference regions (14–17), and in the effects of physical decay and biologic redistribution after administration (14,16,18) and by the use of patient selection with different diagnostic confidences (1–3,15,39). Although these factors may have contributed to some differences between our results and those of previous studies, we nevertheless show that useful semiquantitative data with trends similar to those in the literature can be obtained under these conditions. In addition, because 99mTc-TRODAT-1 provides images that are of good quality, have high target-to-nontarget ratios, and show uneven patterns of dopamine loss in Parkinson’s disease patients (5,15,34), visual interpretation of the images may be a useful supplement for assessing patient status.
CONCLUSION
The findings of this study indicate that 99mTc-TRODAT-1 is a clinically feasible radiopharmaceutical for studying DAT in patients with Parkinson’s disease. The high target-to-noise ratio for 99mTc-TRODAT-1 and significant reduction of DAT binding in patients with mild Parkinson’s disease may permit earlier or even presymptomatic identification of Parkinson’s disease. Our results confirm the potential of using 99mTc-TRODAT-1 for DAT measurement, which is clinically important for the early diagnosis of Parkinson’s disease.
Acknowledgments
The authors thank Li-Mei Wang and Jiang-Cherng Perng for able technical help and Prof. Cheng-Yu Chen, a senior neuroradiologist, for assistance with image integration. This study was supported by the Atomic Energy Council, the National Science Council, and the Ministry of Economical Affairs, R.O.C., under grants N3I07, NSC88-NU-B016-001-NU, and EC-2A-17-0304.
Footnotes
Received Dec. 20, 2000; revision accepted May 14, 2001.
For correspondence or reprints contact: Wen-Sheng Huang, MD, Department of Nuclear Medicine, Tri-Service General Hospital, 325 Section 2, Cherng-kung Rd., Taipei, 114, Taiwan, R.O.C.