Abstract
Biodistribution and dosimetry of 88Y (and equimolar 90Y) Janus-dodecanetetraacetic acid (DOTA) were performed using a three-step pretargeting protocol in BALB/c mice bearing mouse mammary adenocarcinoma (KHJJ) implants. Pretargeting was performed with mouse monoclonal antibody (mAb) 2D12.5 specific for yttrium-DOTA, and the chase was Y-DOTA–human transferrin conjugate. In this article, we report extensive organ dosimetry and the theoretic limits of the radionuclide physical half-life (Tp) for pretargeting. Methods: Organ biodistribution data were obtained from bioassays on tissue taken from tumor mice killed at 3, 24, 48, 72, 96, and 120 h after intravenous injection of 88Y-Janus-DOTA. Uptake and retention of 88Y as a function of time were described by nonlinear least squares fits of the tissue data to multiexponential functions. Radiation dose estimates for equivalent molar amounts of 90Y were subsequently derived from these time-integrated functions. Results: The results were as follows: rapid blood clearance of 88Y-Janus-DOTA; rapid uptake and slow clearance of 88Y-Janus-DOTA from the tumor over 5 d; rapid clearance from all organs and body; largest radiation absorbed dose (AD) per injected dose of 63.52 (cGy/MBq) to tumor; high therapeutic ratios (AD tumor/AD tissue), particularly for blood and bone; and optimal radionuclide Tp range from 30 min to 10 d. Conclusion: Although the absolute concentration of 90Y in the tumor is less using the hapten system than is achieved generally with the chelated radionuclide covalently attached to the mAb, the achievable tumor uptake of radioactivity, coupled with low radioactivity in bone, blood, and other organs, suggests that a three-step pretargeting protocol has considerable promise as a method for 90Y radioimmunotherapy.
Antibodies have been labeled with radioisotopes and used in both the localization and therapy of cancer (1–6). The development of monoclonal antibodies (mAbs) with high specificity for tumor marker antigens has made this approach very attractive, because of the possibility of delivering radionuclides to a target tumor in vivo with great selectivity (1,7).
Human imaging studies have shown that although maximum human tumor concentrations of radiolabeled mAb are achieved in 1 d, the slow pharmacokinetics requires several days for the background to fall sufficiently for sensitive radioimmunoscintigraphy of tumors (8). Waiting for the optimum imaging time is a strategy made possible using larger amounts of longer half-life diagnostic radionuclides, such as 111In (9). With therapeutic radionuclides, however, this long biologic half-life imposes a high radiation burden on sensitive normal tissues, especially bone marrow and gut, from the large amount of retained radioactivity (10,11).
Among the metallic radionuclides for radioimmunotherapy, 90Y is especially attractive because of its availability, pure high-energy β-emission, and high dose yield per picomole (12). However, 90Y-mAb conjugated with conventional bifunctional chelators, such as the cyclic dianhydride of diethylenetriaminepentaacetic acid (DTPA), produces bone marrow toxicity in mice before curative therapeutic levels in the tumor are reached (13).
Moi et al. (14) and Renn and Meares (15) developed a macrocyclic bifunctional chelating agent, 2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA), that holds yttrium with extraordinary stability under physiologic conditions in human serum (16). Stable chelation of 90Y by Janus-DOTA leads to a reduction of free 90Y that potentially could accumulate in bone (17).
Use of a three-step pretargeting technique developed originally for immunoscintigraphy (18) provides a way to get highly selective tumor uptake of 90Y-DOTA with simultaneous minimization of nontarget tissue background (17). Briefly, the antibody and radiolabel are administered separately. The first or pretargeting step consists of administration of mAb to BALB/c mice with KHJJ mouse tumors. Sufficient time (20 h) is allowed for the tumor to equilibrate with blood mAb. At equilibrium, the tumor has as high an mAb concentration as possible. The next step or chase step is the intravenous injection of a large-molecular-weight polyvalent hapten. This procedure leads to the rapid clearance from blood of excess circulating nonradioactive antibody (19). Finally, 1 h after administration of the chase, the radiolabeled hapten–chelate conjugate is given, and mouse organ and tumor assay or whole-body imaging is performed at various times after the addition of the radiolabel (20). Recognizing these principles, Axworthy et al. (21) used the avidin/biotin system to cure human carcinoma xenografts in mice by a single dose of pretargeted 90Y with negligible toxicity.
Substituting the γ-emitting radionuclides 88Y and 111In as tracers for 90Y-Janus-DOTA, we extend our previous analysis (17) to early blood radioactivity levels and a more complete description of the biodistribution and dosimetry of 90Y to tumor, blood, heart, lung, liver, spleen, kidneys, muscle, bone, skin, and gut. 88Y has a physical half-life (Tp) of 106.64 d and decays through both electron capture and positron emission. This decay produces both x-ray and γ-ray emissions, making it suitable for accurate organ assay in a scintillation well counter.
MATERIALS AND METHODS
Organ biodistribution data were obtained from bioassays performed on tissue taken from tumor mice killed at 3, 24, 48, 72, 96, and 120 h after 88Y-Janus-DOTA injection. Uptake and retention of 88Y as a function of time were described by nonlinear least squares fits of the tissue data to multiexponential functions. Radiation dose estimates for equivalent molar amounts of 90Y were derived subsequently from area-under-the-curve (AUC) estimations calculated from these integrated functions (17).
Mouse Tumor Model and Pretargeting Protocol
The KHJJ mouse breast adenocarcinoma was used in these experiments (22). Unlike the more commonly used nude mouse–human tumor system, this model is analogous to tumor imaging in an immunologically competent patient using human mAb. BALB/c mice were used 2 wk after trocar implantation in the flank, at which time the tumors were approximately 0.7 cm in diameter and weighed 558 ± 220 mg (mean ± SD; n = 18).
Pretargeting used three steps. First, 50–100 μg (0.33–0.67 nmol) 2D12.5 were given intravenously to BALB/c mice bearing KHJJ mouse tumors. Enough time (20 h) was allowed for the mAb to leak through the abnormally permeable tumor neovasculature and to equilibrate with the blood mAb to give the maximum tumor concentration. At this time, a multivalent Y-DOTA conjugate of human transferrin (35.4 μg HTr-2IT-BAD-Y, 0.442 nmol HTr, 3.09 nmol BAD-Y: 6/1 hapten/protein) was given intravenously to clear the blood quickly of the excess circulating nonradioactive antibody. One hour after the chase (21 h after mAb), the 88Y-labeled hapten–chelate was given intravenously (33.3 kBq 88Y, 0.744 pmol Y, 0.484 nmol Janus-DOTA).
The equilibrium association constant (Ka), measured by equilibrium dialysis, for the affinity of 2D12.5 and 88Y-DOTA is approximately 108 (17). A previous study in this laboratory (17) with 111In, 88Y, 67Ga, and mono- and divalent haptens using the same pretargeting mAb protocol suggested passive tumor uptake of the antibody through leaky capillaries with no organ uptake, because of the presence of cross-reacting antigen elsewhere in the tissues.
Organ Biodistribution
The tumor mice (n = 3 per group) were killed 3, 24, 48, 72, 96, and 120 h after 88Y-Janus-DOTA injection. The percentage injected dose per gram (%ID/g) of decay-corrected 88Y-labeled hapten was determined in blood, heart, lungs, liver, spleen, kidneys, tumor, muscle, bone and marrow (femur), skin, and gut. To obtain a better estimation of the early blood clearance of the metal–hapten chelate, a second experiment was performed as described previously, substituting 111In-Janus-DOTA for 88Y-Janus-DOTA and sampling at 0.08, 0.17, 0.25, 0.5, 1.0, 1.5, 2.0, and 2.5 h after injection.
Data Analysis
Uptake and retention of the 88Y- or 111In-Janus-DOTA as a function of time were described by iterative nonlinear least squares fits of the %ID/g tumor and organ data to multiexponential functions using SAAM II (SAAM Institute, Inc., Seattle, WA), which is a simulation, analysis, and modeling software package.
If fj(t) is defined as the %ID/g of metal-chelate as a function of time, then:
 Eq. 1
where Aj (%ID/g) and λj (biologic uptake or decay rate, time−1) are the fitted parameters.
Eq. 1
where Aj (%ID/g) and λj (biologic uptake or decay rate, time−1) are the fitted parameters.
Absorbed Dose Estimates
Radiation absorbed dose (AD) estimates for equivalent molar amounts of 90Y were derived subsequently from AUC estimates. These were derived from the fitted exponential functions describing the biodistribution of the injected 88Y activity and the 90Y physical decay constant. If fh(t) is defined as 90Y MBq/kg as a function of time, then:
 Eq. 2
where c1 is a constant for the conversion of %ID/g to 90Y MBq/kg and λp is the 90Y physical decay constant (time−1). Substituting ΣAj exp(−λjt) for fj(t) gives:
Eq. 2
where c1 is a constant for the conversion of %ID/g to 90Y MBq/kg and λp is the 90Y physical decay constant (time−1). Substituting ΣAj exp(−λjt) for fj(t) gives:
 Eq. 3
and
Eq. 3
and
 Eq. 4
Eq. 4
If the AUC from time zero to infinity is defined as the integral from zero to infinity, then:
 Eq. 5
or
Eq. 5
or
 Eq. 6
which, when integrated, becomes:
Eq. 6
which, when integrated, becomes:
 Eq. 7
Eq. 7
Finally, if AD is defined as the product of the AUC from zero to infinity and the energy constant, then:
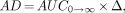 Eq. 8
where Δ is the 90Y energy constant (1.49 × 10−5 cGy × kg/MBq × s). Then:
Eq. 8
where Δ is the 90Y energy constant (1.49 × 10−5 cGy × kg/MBq × s). Then:
 Eq. 9
Eq. 9
These calculations do not consider the reduction of the dose caused by the escape of energy from the small mouse organs or the effect of such escaped energy on the other organs. Because we are dealing primarily with pure β-emitters, this situation will have little implication for human dosimetry in treatment situations.
Statistics
The AD estimates for the tumor and organs were compared by ANOVA and the Dunnett multiple comparison test, which compared the AD of the tumor to the AD of blood and other tissue (23). In all cases, P < 0.01 was considered statistically significant.
RESULTS
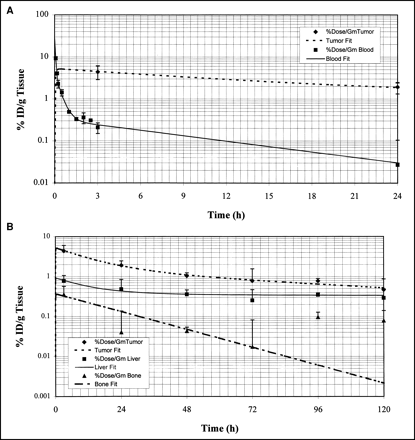
Figure 1A is a plot of the %ID/g blood versus time after intravenous injection of 111In- and 88Y-Janus-DOTA 1 h after injection of the polyvalent hapten–protein conjugate. The data from 0 to 2.5 h are from 111In-Janus-DOTA, and those from 3 to 24 h are from 88Y-Janus-DOTA. The data were fitted to the following triexponential function:
 Eq. 10
Eq. 10
 where Aj (%ID/g) and λj (h−1) were the fitted parameters.
where Aj (%ID/g) and λj (h−1) were the fitted parameters.
(A) Semilog plots of blood (▪) clearance of both 111In- (0.08–2.5 h) and 88Y-Janus-DOTA, and tumor (⧫) uptake and clearance of 88Y-Janus-DOTA. Note predicted rapid uptake into tumor as blood levels decline rapidly as metal-Janus-DOTA is quickly distributed into interstitial volume. (B) Semilog plots of %ID/g for tumor (⧫), liver (▪), and bone (▴) predicted uptake and clearance of 88Y-Janus-DOTA. Note much higher initial accumulation in tumor compared with bone and liver.
The initial blood concentration, C0, was 30.06 ± 17.95 %ID/g (mean ± SD), and the estimated volume of distribution (VD) was 3.3 mL. Both 111In- and 88Y-Janus-DOTA were cleared rapidly from the blood; only about 0.2 %ID/g remained at 3 h, and <0.03 %ID/g remained at 24 h (Table 1; Fig. 1A).
Tumor and Organ 5-Day Biodistribution Data for 88Y-Janus-DOTA
The uptake and clearance of 88Y-Janus-DOTA from the tumor and organs over 5 d is shown in Figure 1B. The tumor data were fitted to the following triexponential function:
 Eq. 11
Eq. 11
 where A3 = −(A1 + A2) such that ΣAj = 0 at t = 0, and the initial estimate for λ3 (uptake phase) was obtained from the λ1 (distribution phase) of the %ID/g blood versus time curve.
where A3 = −(A1 + A2) such that ΣAj = 0 at t = 0, and the initial estimate for λ3 (uptake phase) was obtained from the λ1 (distribution phase) of the %ID/g blood versus time curve.
Clearance of the metal-DOTA from the tumor was slow compared with the rapid blood clearance. The metal-chelate was bound to the pretargeted antibody in the tumor with 4.44 and 1.93 %ID/g at 3 and 24 h, respectively. This result gave tumor-to-blood ratios of approximately 20:1 and 60:1 at 3 and 24 h, respectively.
The biodistribution data for the remaining organs were fitted to multiexponential equations as above. Figure 1B shows the %ID/g 88Y-Janus-DOTA versus time for tumor, liver, and bone. Values of the %ID/g 88Y-Janus-DOTA at 3, 24, 48, 72, 96, and 120 h for the tumor and organs are listed in Table 1.
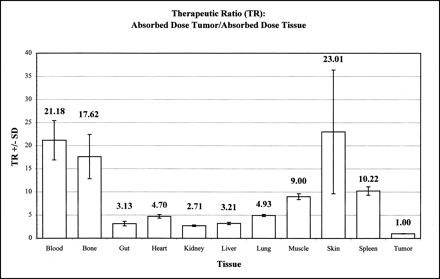
Table 2 summarizes the tumor and organ time-integrated radioactivity (90Y MBq × s/kg) and AD (cGy) for 90Y-Janus-DOTA obtained from the fitted exponential functions describing the biodistribution of the injected dose. A summary of the AD (cGy) per megabecquerel injected 90Y for tumor and organs is shown in Figure 2. Note that the tumor had a much greater AD per injected dose (cGy/MBq), which was highly significant compared with all other tissue (P < 0.01, ANOVA, Dunnett). The therapeutic ratios (TRs) defined as AD tumor/AD tissue are shown in Figure 3. Note the large TRs for both blood and bone.
Summary of AD (cGy)/injected dose (MBq) 90Y for tumor and organs. The tumor had largest AD compared with all other tissue (P < 0.01, ANOVA, Dunnett).
Summary of TRs calculated as AD tumor/AD tissue. Note large TRs for blood and bone.
Tumor and Organ Time-Integrated Activity and Absorbed Dose for 90Y-Janus-DOTA
DISCUSSION
The mAb from clone 2D12.5 was used because it had the highest 24-h whole-body retention of 88Y-DOTA caused by a high affinity for Y(III)-DOTA (Ka = 108 mol/L). After intravenous injection in mice, uncomplexed 88Y-, 111In-, and 67Ga-labeled DOTA were all excreted rapidly in the urine. The half-life for elimination was approximately 40 min; >93% of the injected dose was excreted by the kidneys in 24 h (17).
Our model showed a rapid uptake of the 88Y-Janus-DOTA (Cmax at tmax of 0.4 h) into the leaky neovasculature of the tumor as the injected metal-DOTA was distributed rapidly into the interstitial water (Fig. 1B). Figure 1A is a plot of the %ID/g blood versus time after intravenous injection of 111In- and 88Y-Janus-DOTA 1 h after injection of the polyvalent hapten–protein conjugate. Rapid blood clearance was seen with both 88Y- and 111In-Janus-DOTA.
Previously, we had shown that rapid lowering (chase) of a long-circulating protein was possible by the intravenous injection of a specific antibody (19). In these experiments, the chase of 2D12.5 by injection of the polyvalent hapten–protein conjugate also rapidly lowered the circulating mAb concentration. This procedure had a striking effect on the whole-body 24-h retention of the metal-DOTA injected soon after. When the chase was administered to 20-h pretargeted mice 1 h before 88Y-DOTA injection, whole-body retention of the metal-chelate fell from >80% (no chase) to approximately 10% at 3 h and approximately 5% at 24 h after injection of the tracer.
As a matching isotope for 90Y, 111In has been the nuclide of choice because of the similarities in its metabolic handling and coordination chemistry (24). Comparative studies with 111In or 90Y radiolabeled compounds have shown similar pharmacokinetic behavior of the two species (25). Moreover, nitrobenzyl-DOTA binds both yttrium and indium with high stability (26). On the basis of these reports showing that the physiochemic properties of the metal-DOTA are determined primarily by the chelate and not the metal ligand, the 111In- and 88Y-DOTA biodistribution data were pooled.
Pretargeting involves the administration of a long-circulating targeted macromolecule (mAb) having a high-affinity noncovalent binding site for a small, rapidly excreted effector molecule, which is given after the mAb has concentrated in the targeted tumor (20). Efficient removal of the hapten-binding macromolecule (mAb) from the circulation with a polyvalent chase macromolecule before giving the effector molecule greatly improves the target-to-blood ratio. Pretargeting without the chase step requires a long waiting period for the blood mAb concentration to fall to very low levels, because even small amounts of mAb remaining in the blood will immediately bind effector molecules on injection. To ensure free effector molecules for diffusion into the tumor and filtration and excretion by the kidneys, circulating mAb must be either saturated or removed. The aggregated mAb produced by cross-linking with the chase in the circulation is endocytosed rapidly by the reticuloendothelial cells, mostly in the liver (18). The intracellular location of the endocytosed mAb prevents the access and binding of a subsequently injected hydrophilic effector molecule. The chase quickly reduces the blood mAb to a very low level so that radioactivity can be administered within 1 h (27).
After injection of the effector molecule (radiolabeled hapten) 1 h after the chase molecule, maximum tumor concentration and tumor-to-normal-tissue ratios were achieved rapidly, and radioactivity was retained in the tumor for several days. The rapid elimination of unlocalized radioactivity greatly decreased radiation exposure to normal tissue, especially the bone marrow (Figs. 1B and 2). Thus, the pharmacokinetics of the effector molecules gave fast tumor uptake (minutes), slow tumor washout (days), and relatively fast washout from most organs (Figs. 1B and 2, Table 1). This result provided high tumor-to-background ratios and high TRs, which will improve both imaging and radioimmunotherapy (Figs. 2 and 3).
Rapid diffusion, exclusive renal excretion, and extracellular fluid distribution are among the chemical and physiologic properties of an effector molecule that are necessary for achieving the best pretargeting results (26). Our experimental determination of the apparent VD of 3.3 mL for the metal-DOTA was consistent with literature estimates of the extracellular fluid in the mouse (28). This result, combined with the net negative charge and hydrophilic nature of the metal-hapten, suggested that the metal-DOTA was confined essentially to the extracellular volume with very little intracellular uptake (29).
Rapid uptake of the metal-DOTA by both tumor and organs seen in our model (Figs. 1A and B) with a Cmax at approximately 0.4 h was consistent with rapid distribution of the metal-DOTA into the interstitial water. Clearance of the metal-DOTA from the tumor was slow compared with that of blood, because the metal-DOTA was bound to the pretargeted antibody (Fig. 1A).
From the tumor and organ time-integrated radioactivity (90Y MBq × s/kg) and AD (cGy) for 90Y-Janus-DOTA, the tumor had the largest AD compared with all other tissue (Fig. 2, Table 2). Moreover, TRs were >2.5/1 for all measured tissue (Fig. 3); TRs for both blood (21/1) and bone (18/1) were particularly dramatic. The tumor-to-blood TR of >20/1 was much greater than the 2–3/1 obtained with directly labeled mAb (1). This large gain in the tumor-to-blood TR is highly desirable for both radioimmunodetection and radioimmunotherapy.
Radiation dose is related to the effective retention of activity in tissue (Te), reflecting both radioactive decay and biologic clearance processes (30). Biologic retention and clearance values are best characterized by the AUC (%ID × h/g tissue) obtained from the integration of the equation describing the %ID/g tissue (obtained from a suitable tracer) as a function of time (Eq. 1) and is given by the following relationship:
 Eq. 12
where Aj (%ID/g tissue) and λj (biologic decay and uptake rate constants, h−1) are obtained from the fit of Equation 1. Cumulative activity in tissue is derived from the decay-corrected tissue count data and Tp of the radionuclide and is best characterized by the AUC (MBq × s/kg tissue) obtained from the integration of Equation 4. Cumulated activity in tissue is given by the following relationship:
Eq. 12
where Aj (%ID/g tissue) and λj (biologic decay and uptake rate constants, h−1) are obtained from the fit of Equation 1. Cumulative activity in tissue is derived from the decay-corrected tissue count data and Tp of the radionuclide and is best characterized by the AUC (MBq × s/kg tissue) obtained from the integration of Equation 4. Cumulated activity in tissue is given by the following relationship:
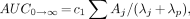 Eq. 13
where c1 is a constant for the conversion of %ID/g to MBq/kg and λp is the radionuclide decay constant (time−1) (Eq. 7).
Eq. 13
where c1 is a constant for the conversion of %ID/g to MBq/kg and λp is the radionuclide decay constant (time−1) (Eq. 7).
As a first approximation, the AD is related to the cumulative activity in a specific tissue (Eq. 8). For several reasons, the dose is not directly proportional to cumulated activity. Some of the energy emitted from radionuclides in an organ or tissue may be imparted to more distant organs or may completely escape the body. Moreover, accurate AD calculations also depend on the size and shape of the organ and the density and atomic composition of the tissue (30). In a mouse model, Hui et al. (31) and Beatty et al. (32) showed that the dosimetry calculation model, using the standard assumption for humans that all β-energy is deposited within the organs or tumor in which it is localized, is inadequate for determining the β-AD for mouse organs. Because the tumors in our mouse model weighed significantly more, we estimated the self-absorbed fraction to be approximately 0.5 for our tumors. Thus, the actual dose to our tumors is probably closer to half the value we calculated using standard assumptions. On the other hand, we did not consider the dose caused by β-particles entering from adjacent tissue or as circulating activity, which is lower with pretargeting than with conventional radioimmunotherapy. Although the mouse dosimetry is overestimated, the uncorrected values should apply to larger human organs (17).
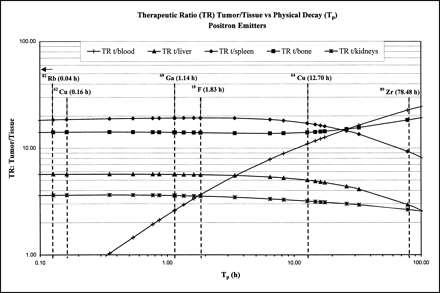
It is likely, as shown for 111In, 88Y, and 90Y, that the biodistribution and biologic half-life (Tb) of many other metal-DOTA chelates will be very similar if not identical (33). Thus, the relative AD for each radiometal to the organs and the tumor should be directly proportional to the Tp for that radionuclide. Plotting several TRs as a function of Tp (Figs. 4 and 5) for various imaging and therapeutic radionuclides, we notice that the tumor-to-liver TR is <1 for radionuclides with half-lives >10 d. The reason for this result is suggested by a careful analysis of the biodistribution data for the liver. Although the absolute %ID/g is small for the liver, the radionuclide–DOTA–mAb complex is not cleared readily and thus has a very long biologic half-life compared with other tissue (Fig. 1B). Thus, for therapeutic radionuclides with TP > 10 d, the Te of the complex in the liver is quite pronounced, with more energy imparted to the liver than to the tumor. Similarly, for radionuclides with TP < 1 h, the TR decreases rapidly, especially for the blood and gut.
Summary plot of several tumor-to-tissue TRs vs. Tp for select number of positron-emitting radionuclides.
Summary plot of several tumor-to-tissue TRs vs. Tp for various therapeutic radionuclides.
CONCLUSION
The high tumor uptake of 90Y-DOTA and subsequent high radioactivity, coupled with low radioactivity in bone, blood, and other organs, suggests that a three-step pretargeting protocol has considerable promise as a method for 90Y-radioimmunotherapy.
Acknowledgments
This study was supported in part by the Medical Research Service of the Department of Veterans Affairs (Program 821 Merit Review and special program support) (DAG and MH) and PHS grant numbers CA 28343 and CA 48282 (DAG) and CA 16861 (CFM).
Footnotes
Received May 26, 2000; revision accepted Dec. 11, 2000.
For correspondence or reprints contact: Stephen P. Lubic, PhD, 1316 Alderbrook Lane, San Jose, CA 95129.